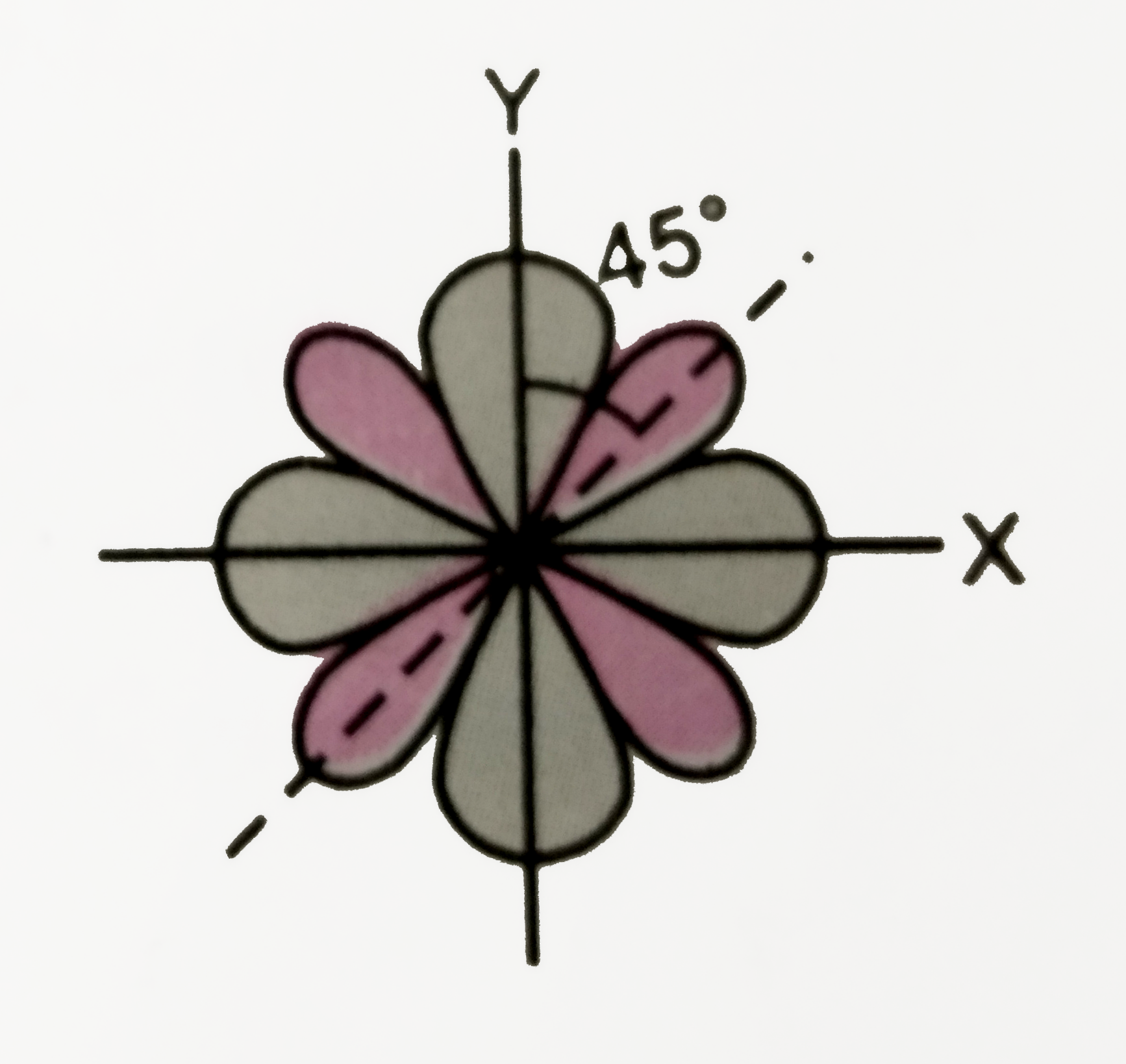
Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
PRADEEP|Exercise NCERT Questions And Exercises with Answers|67 VideosSTRUCTURE OF ATOM
PRADEEP|Exercise NCERT Exemplar Problems With Answers, Hints and Solution (I. Multiple Choice Questions)|16 VideosSTRUCTURE OF ATOM
PRADEEP|Exercise Test Your Grip (Fill in the Blanks)|24 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (ASSERTION-REASON)|17 VideosTHERMODYNAMICS
PRADEEP|Exercise MULTIPLE CHOICE QUESTION ( BASED ON PRACTICAL CHEMISTRY)|3 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-STRUCTURE OF ATOM-Conceptual Questions
- Name the quantum number which does not follow from the solution of Sc...
Text Solution
|
- What is the difference between the notations l and L ?
Text Solution
|
- Which of the four quantum number (n, l, m(l), m(s)) determine (a) the ...
Text Solution
|
- Why Pauli exlusion principle is called exlusion principle ?
Text Solution
|
- At what distance is the radial probability maximum for 1s orbital ? Wh...
Text Solution
|
- Do atomic orbitals have sharp boundaries ? Explain why or why not ? or...
Text Solution
|
- Draw the shapes (boundary surfaces) of the following orbitals: (a) 2...
Text Solution
|
- Discuss the similarities and differences between a 1s and 2s orbital.
Text Solution
|
- How many radial/spherical nodes will be present in the 5f orbital ?
Text Solution
|
- (i) What is common between d9xy) and d(x^(2) - y^(2)) orbitals ? (ii) ...
Text Solution
|
- For each of the following pair of hydrogen orbitals, indicate which is...
Text Solution
|
- Which orbital in each of the following pairs is lower in energy in a m...
Text Solution
|
- Why Hund's rule is called rule of maximum multiplicity ?
Text Solution
|
- How many electrons in sulphur (Z = 16) can have n + l = 3 ?
Text Solution
|
- The 4f subshell of an atom contains 10 electrons. What is the maximum ...
Text Solution
|
- Which out of Cu^(2+), Fe^(2+) and Cr^(3+) has highest paramagnetism an...
Text Solution
|
- What is the maximum number of electrons that can be present in an atom...
Text Solution
|
- One unpaired electron in an atom distributes a magnetic moment of 1.1 ...
Text Solution
|
- The ground-state electronic configurations listed here are incorrect. ...
Text Solution
|
- What is the deviation from Aufbau Principle in case of electronic conf...
Text Solution
|