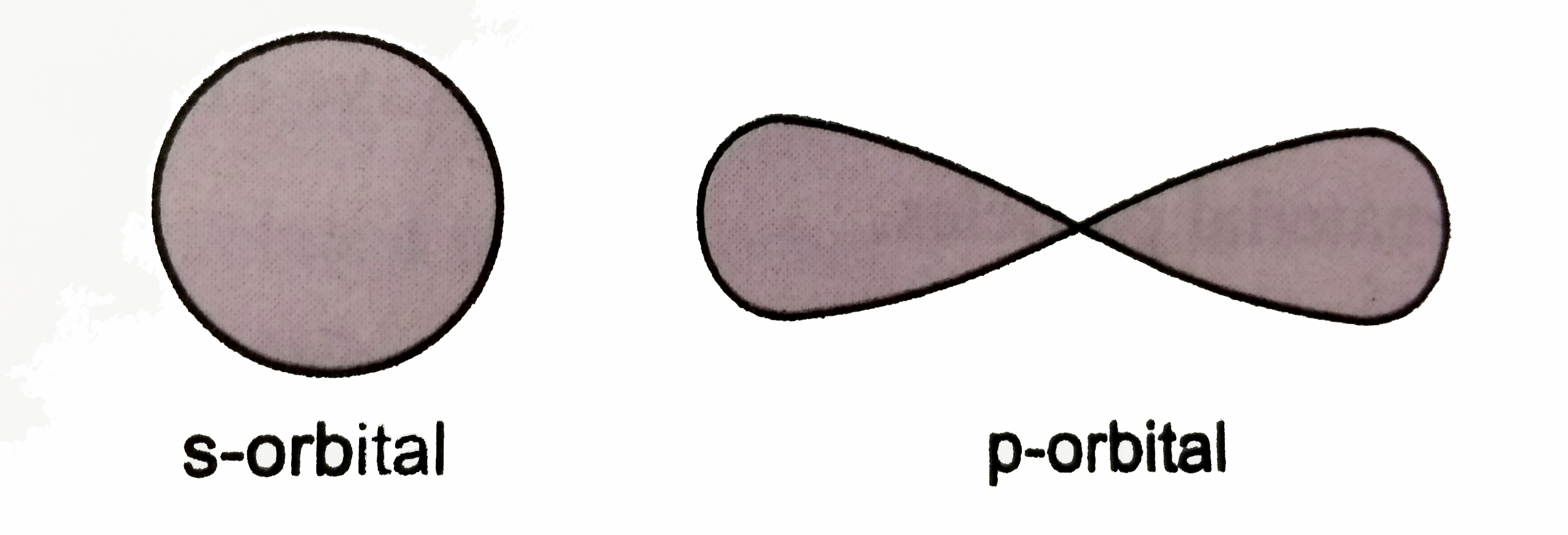
Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
PRADEEP|Exercise Additional Questions (Short Answer question)|47 VideosSTRUCTURE OF ATOM
PRADEEP|Exercise Additional Questions (Long Answer Questions)|16 VideosSTRUCTURE OF ATOM
PRADEEP|Exercise NCERT Exemplar Problems With Answers, Hints and Solution (Long Answer Questions)|5 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (ASSERTION-REASON)|17 VideosTHERMODYNAMICS
PRADEEP|Exercise MULTIPLE CHOICE QUESTION ( BASED ON PRACTICAL CHEMISTRY)|3 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-STRUCTURE OF ATOM-Additional Questions (Very Short Answer Questions)
- What values of m are permitted for electron having angular quantum ...
Text Solution
|
- What value are permitted for the angular momentum quantum number l for...
Text Solution
|
- What are the values of n, l and m for 2p(x) and 3p(z) orbitals ?
Text Solution
|
- Which orbitals is non-directional ?
Text Solution
|
- How many orbitals do you expect to be present in the 5th shell ?
Text Solution
|
- Which d-orbital does not have four lobes and what is its shape called ...
Text Solution
|
- (a) What is radial probability distribution curve ? Draw radial probab...
Text Solution
|
- What is the physical significance of the lines in the following depict...
Text Solution
|
- Draw the shapes of various p and d orbitals.
Text Solution
|
- What will be the order of energy levels 3s, 3p and 3d in case of H-ato...
Text Solution
|
- What are degenerate orbitals ?
Text Solution
|
- Which element has only one electron in the d-orbitals ?
Text Solution
|
- Write the electronic configuartion of Cu^(+) (At. No. of Cu= 29)
Text Solution
|
- Copper (I) is diamagnetic while copper (II) is paramagnetic . Explain...
Text Solution
|
- Why is the electronic configuration 1s^(2) 2s^(2) 2p(x)^(2) 2p(y)^(1) ...
Text Solution
|
- What are the possible values of principal (n) and azimuthal (l) quantu...
Text Solution
|
- Out of Fe^(2+) and Fe^(3+) which is more paramagnetic and why?
Text Solution
|
- Write the electronic configuration of a divalent ion of a coinage meta...
Text Solution
|
- By what name are the following principles known ? (i) Electrons with...
Text Solution
|
- What is the nuclear radius of an atom whose mass number is 125 ?
Text Solution
|