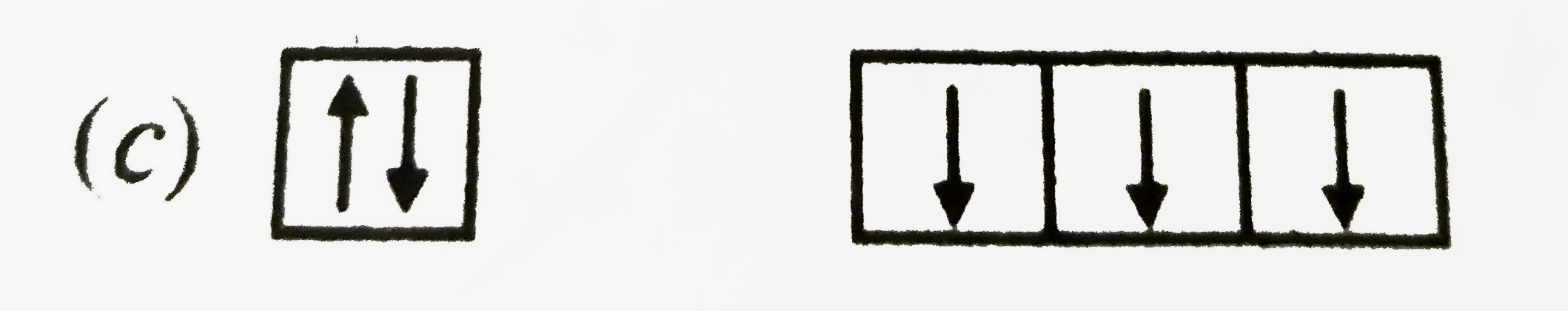A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
PRADEEP|Exercise Competition Focus (JEE (Main and Advanced)/Medical Entrance (II. Multiple Choice Question)|9 VideosSTRUCTURE OF ATOM
PRADEEP|Exercise Competition Focus (JEE (Main and Advanced)/Medical Entrance (III. Multiple Choice Question) (Based on Comprehension)|20 VideosSTRUCTURE OF ATOM
PRADEEP|Exercise Analytical Questions And Problems with Answers/solutions (Problems)|18 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (ASSERTION-REASON)|17 VideosTHERMODYNAMICS
PRADEEP|Exercise MULTIPLE CHOICE QUESTION ( BASED ON PRACTICAL CHEMISTRY)|3 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-STRUCTURE OF ATOM-Competition Focus (JEE (Main and Advanced)/Medical Entrance (I. Multiple Choice Question) With one correct Answer
- What would be the IUPAC name for the element with atomic number ...
Text Solution
|
- Arrabge in decreasing order, the energy of 2s orbital in the following...
Text Solution
|
- The orbital diagram in which both the pauli's exclusion principal and ...
Text Solution
|
- Which is the correct order of increasing energy of the listed orbitals...
Text Solution
|
- If n = 6, the correct sequence for filling of electrons will be.
Text Solution
|
- The number of d-electrons in Fe^(2+)(Z=26) is not equal to the number...
Text Solution
|
- The electronic, identified by quantum numbers n and l, (i) n = 4, l = ...
Text Solution
|
- What is the maximum number of electrons which can be accommodated in a...
Text Solution
|
- If n = 3, l = 0, and m = 0, then the atomic number is
Text Solution
|
- How many electron in an atom with atomic number 105 can have (n + l)...
Text Solution
|
- According to (n+l) rule after completing 'np' level the electron enter...
Text Solution
|
- The correct set of four quantum numbers for the valence electron of ru...
Text Solution
|
- The set of quantum number for the 19^(th) electrons in chromium is -
Text Solution
|
- In Cu (At. no. = 29)
Text Solution
|
- In the Bohr's orbit, what is the ratio of total kinetic energy and tot...
Text Solution
|
- A proton is about 1840 times heavier than an electron. When it is acce...
Text Solution
|
- Time taken by an electrons to complete one revolution in the Bohr orbi...
Text Solution
|
- Which is a wrong statement ?
Text Solution
|
- Which of the following has maximum spin ?
Text Solution
|
- A gas absorbs a photon of 355 nm and emits at two wavelengths . If on...
Text Solution
|