Text Solution
Verified by Experts
Topper's Solved these Questions
S-BLOCK ELEMENTS (ALKALI AND ALKALINE EARTH METALS)
PRADEEP|Exercise Additional Questions (Very Short Answer Questions) Group 1 Elements - Alkali Metals|16 VideosS-BLOCK ELEMENTS (ALKALI AND ALKALINE EARTH METALS)
PRADEEP|Exercise Additional Questions (Very Short Answer Questions) Group 2 Elements - Alkaline Earth Metals|17 VideosS-BLOCK ELEMENTS (ALKALI AND ALKALINE EARTH METALS)
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS , HINTS AND SOLUTIONS (Assertion and Reason Type Questions)|2 VideosREDOX REACTIONS
PRADEEP|Exercise Assertion reason type question|16 VideosSOLID STATE
PRADEEP|Exercise Advanced problems|19 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-S-BLOCK ELEMENTS (ALKALI AND ALKALINE EARTH METALS) -NCERT EXEMPLAR PROBLEMS WITH ANSWERS , HINTS AND SOLUTIONS (Long Answer Questions)
- The s-block elements are characterised by their larger atomic sizes, l...
Text Solution
|
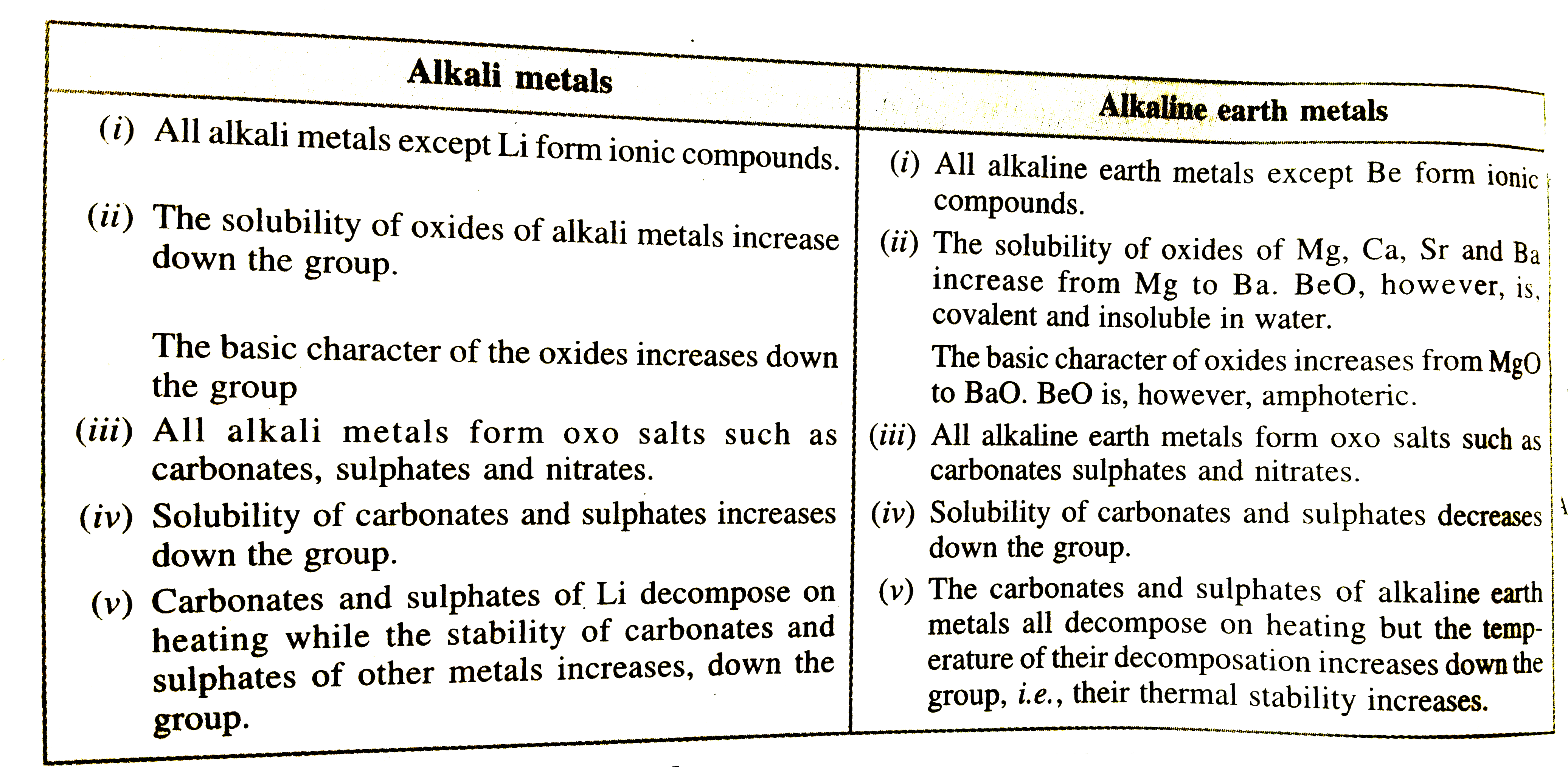
- Present a comparative account of the alkali and alkaline earth metals ...
Text Solution
|
- When a metal of group 1 was dissolved in liquid ammonia, the following...
Text Solution
|
- The stability of peroxide and superoxide of alkali metals increase as ...
Text Solution
|
- When water is added to compound (A) of calcium, solution of compound (...
Text Solution
|
- Lithium hydride can be used to prepare other useful hydrides. Berylliu...
Text Solution
|
- An element of group 2 forms covalent oxide which is amphoteric in natu...
Text Solution
|
- Ions of an element of group 1 participate in the transmission of nerve...
Text Solution
|
- Why does sodium impart yellow colour in the flame ?
Text Solution
|