Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROCARBONS
PRADEEP|Exercise PROBLEMS FOR PRACTICE|3 VideosHYDROCARBONS
PRADEEP|Exercise TEST YOUR GRIP (MULTIPLE CHOICE )|26 VideosHYDROCARBONS
PRADEEP|Exercise CURIOSITY QUESTIONS|4 VideosEQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)|10 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HYDROCARBONS-ADVANCED PROBLEMS FOR COMPETITION
- An organic compound [A] C(6)H(10), on reduction first gives [B] C(6)H(...
Text Solution
|
- A conjugated alkediene having molecular formula C13H22 on ozonolysis y...
Text Solution
|
- A chloro compound with M.F C3H3Cl shows the following properties (i)...
Text Solution
|
- An unsaturated hydrocarbon (A), C6H(10), readily gives (B) on treatmen...
Text Solution
|
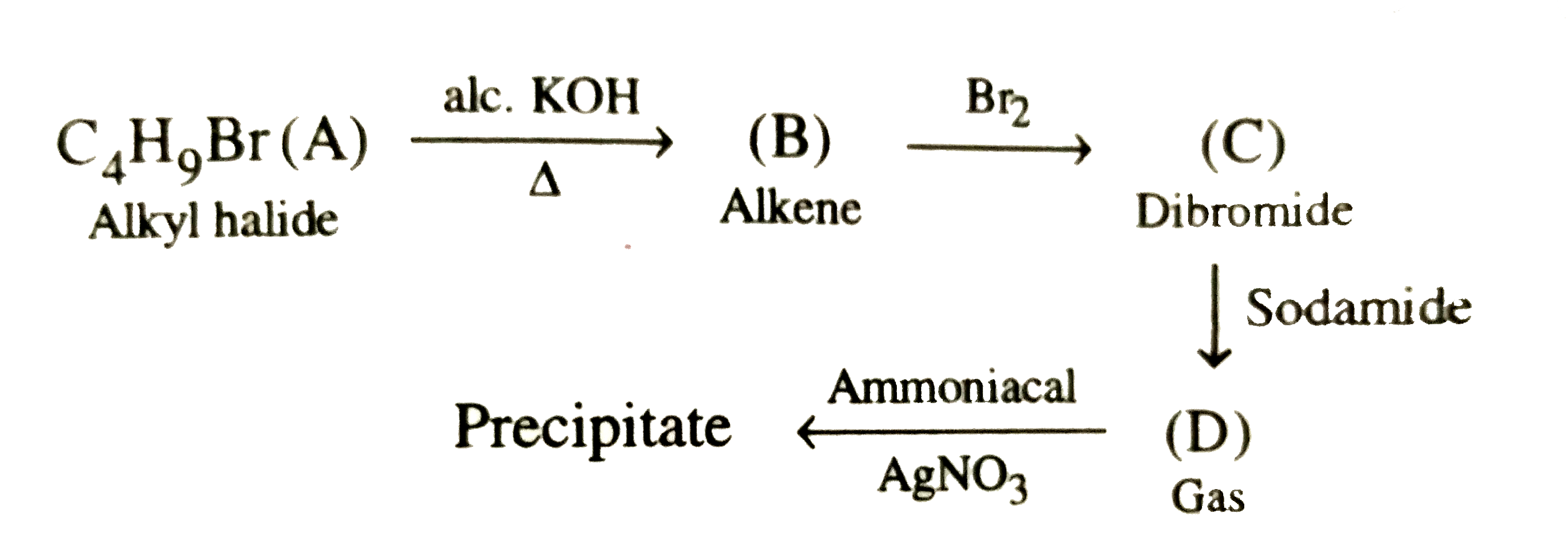
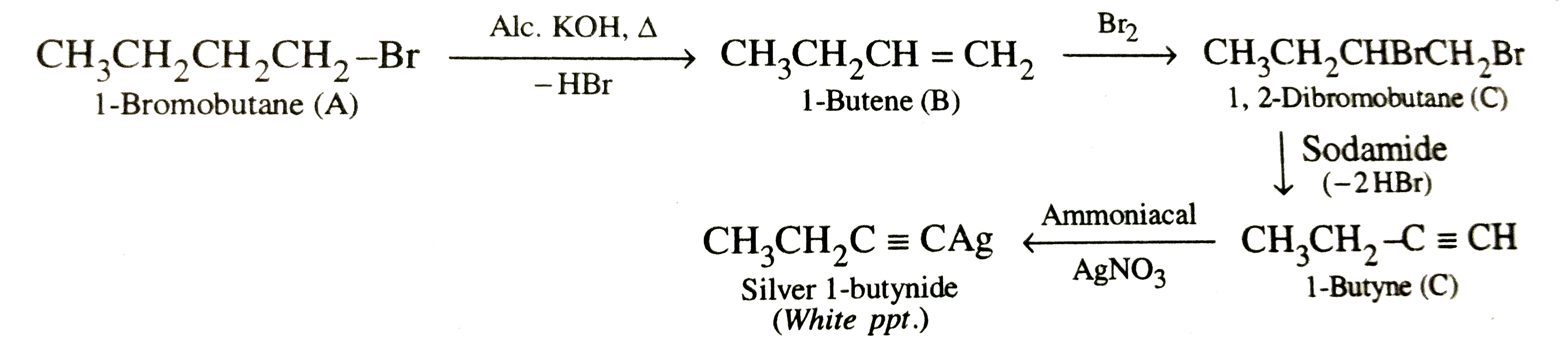
- Primary alkyl halide C(4)H(9)Br (a) reacted with alcoholic KOH to give...
Text Solution
|