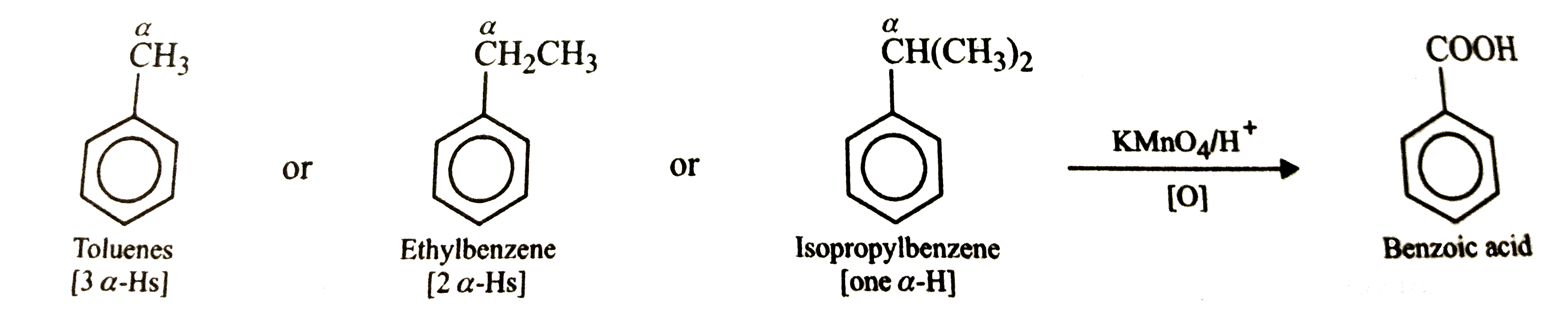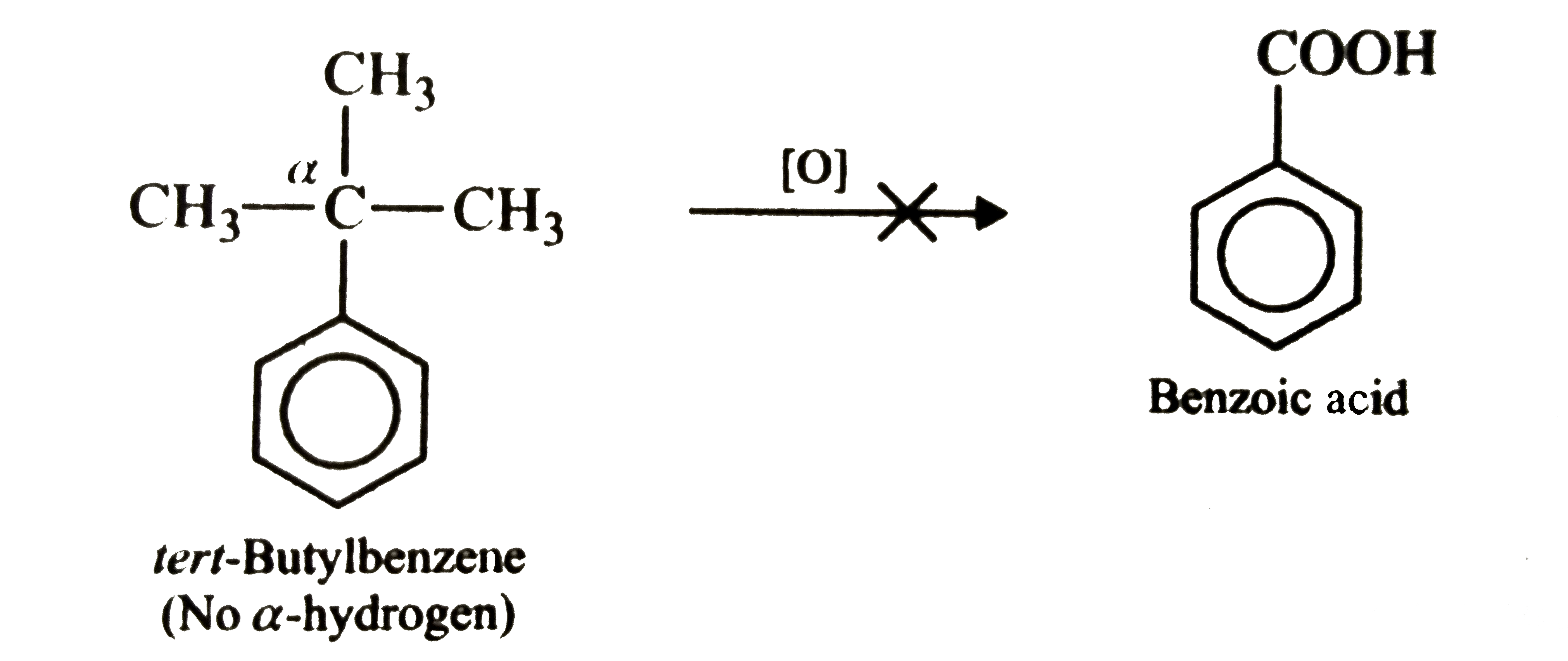Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROCARBONS
PRADEEP|Exercise NCERT QUESTION AND EXERCISES WITH ANSWERS|14 VideosHYDROCARBONS
PRADEEP|Exercise NCERT QUESTION AND EXERCISES WITH ANSWERS (NCERT EXCERCISES)|25 VideosHYDROCARBONS
PRADEEP|Exercise TEST YOUR GRIP (FILL IN THE BLANKS )|20 VideosEQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)|10 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HYDROCARBONS-CONCEPTUAL Questions
- a. Why are alkanes inert ? b. Why the (C----C) bond rather than (C...
Text Solution
|
- Is it possible to isolate pure staggered ethane or pure eclipsed ethan...
Text Solution
|
- Dehydration of alcohol to form an alkene is always carried out with co...
Text Solution
|
- Dehydration of 1-butanol or 2-butanol with conc. H2SO4 always gives th...
Text Solution
|
- Predict the major product of the following reaction: C(6)H(6)+(CH(3))(...
Text Solution
|
- Identify the organic products obtained in the following reaction :
Text Solution
|
- Predict the products of the following reaction
Text Solution
|
- Complete the following reactions with appropriate structures of produc...
Text Solution
|
- How will you prepare 3-methylbut-1-yne by starting with ethyne ?
Text Solution
|
- Predict the major product in the following reaction : R-C-=C-R under...
Text Solution
|
- Identify the product in the reaction PhC-=CMe overset(H3O^(+),Hg^(2+)?...
Text Solution
|
- How will you prepare (i) cis-pent-2-ene and trans-pent-2-ene by starti...
Text Solution
|
- Starting with ethyne, how will you prepare pentan-2-one ?
Text Solution
|
- How will you separate a mixture of ethane, ethylene and acetylene ?
Text Solution
|
- Give reasons for the following: CH2=CH^(Θ) is more basic than HC-=C^...
Text Solution
|
- Predict which of the following systems would be aromatic and why ?
Text Solution
|
- Ozonolysis of mesitylene gives
Text Solution
|
- Write the major product in each case
Text Solution
|
- Tert-Butylbenzene does not benzoic acid on oxidation with acidic KMnO(...
Text Solution
|
- How will you explain the directive influence of (i)-CH=CH2 and (ii...
Text Solution
|