Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE|Exercise SECTION-J AAKASH CHALLENGERS QUESTIONS|10 VideosORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE|Exercise TRY YOURSELF|28 VideosORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE|Exercise SECTION-H MULTIPLE TRUE-FALSE TYPE QUESTION|2 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
AAKASH INSTITUTE|Exercise Assignment(Section-D)(Assertion - Reason Type Questions)|12 VideosPOLYMERS
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION-D)|13 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES-SECTION-I SUBJECTIVE TYPE QUESTIONS
- Rank the given species in the increasing order of water solubility
Text Solution
|
- The given six compounds are similarly sized, very similar molecular we...
Text Solution
|
- For the given pair of compounds, identify the compound you expect to h...
Text Solution
|
- Which of the following reactive intermediate is more stable and why?
Text Solution
|
- The structure of triphenylmethyl cation is given below. This is very s...
Text Solution
|
- For the dehydration reaction.
Text Solution
|
- Which of the following carbanion is less stable and why?
Text Solution
|
- Aromatic amines are weakly basic, whereas is strongly basic, explain ...
Text Solution
|
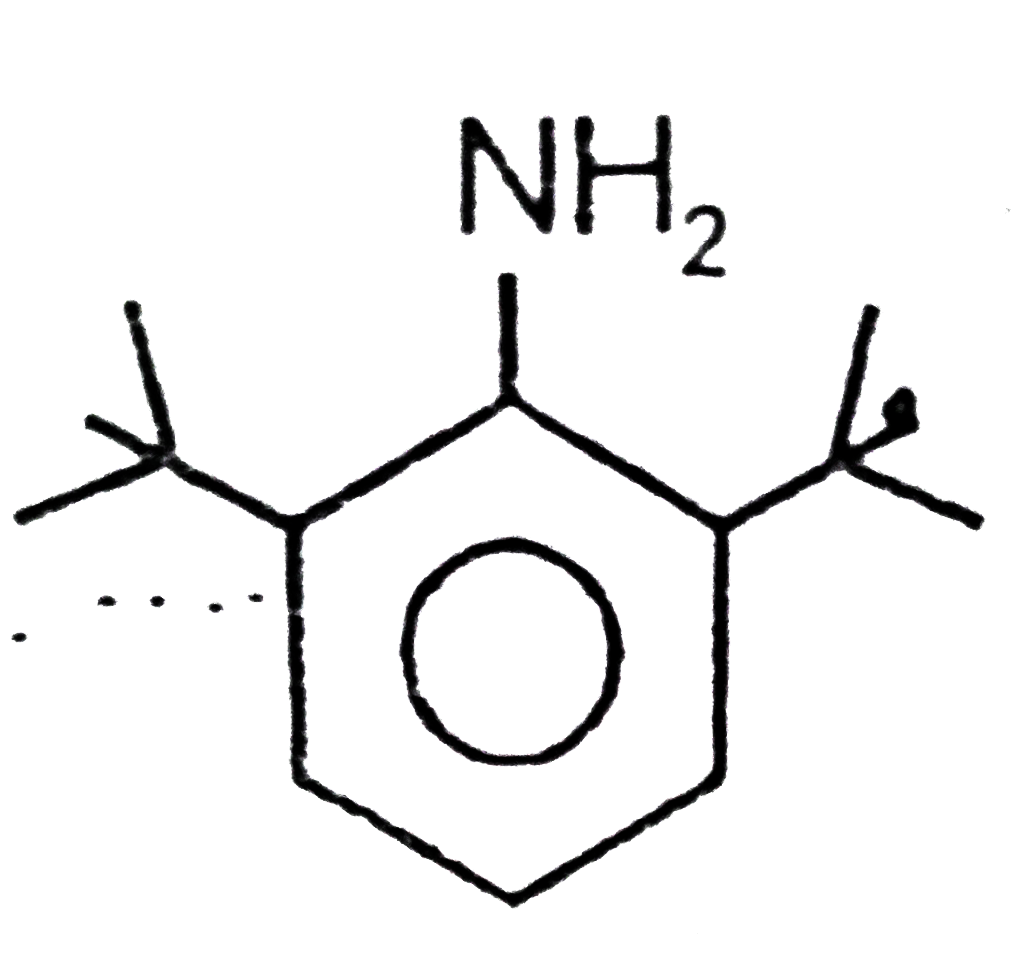 is strongly basic, explain this unusual behaviour.
is strongly basic, explain this unusual behaviour.