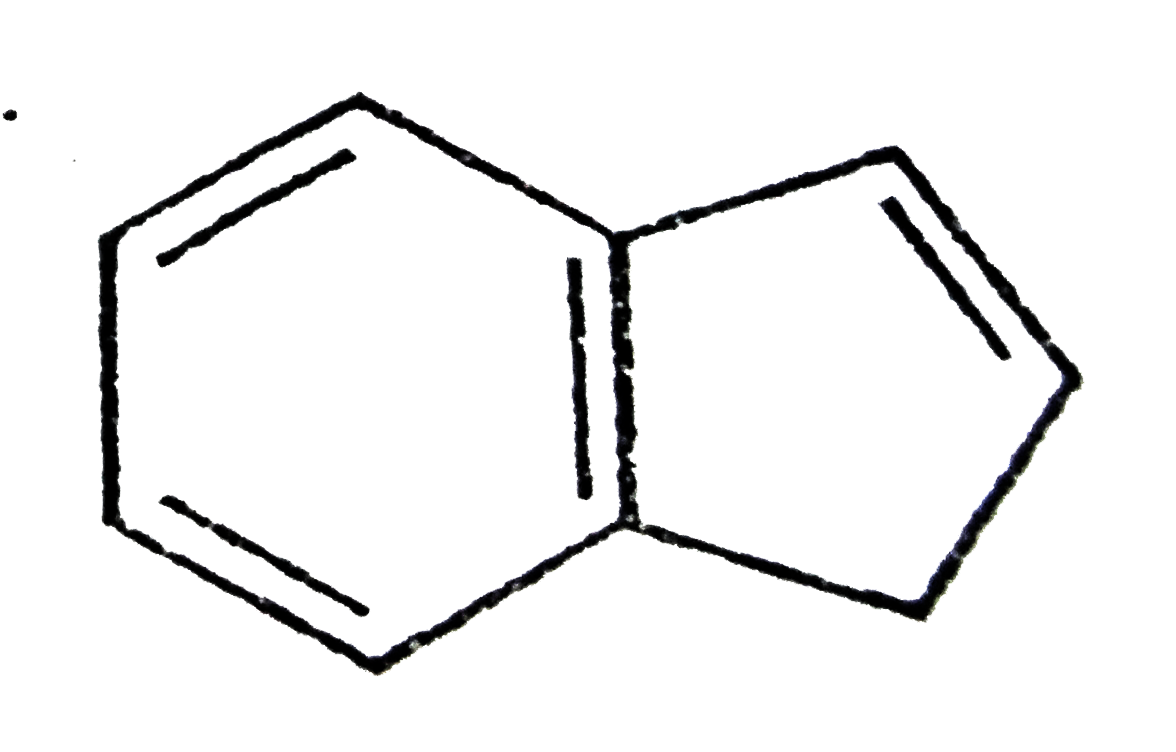
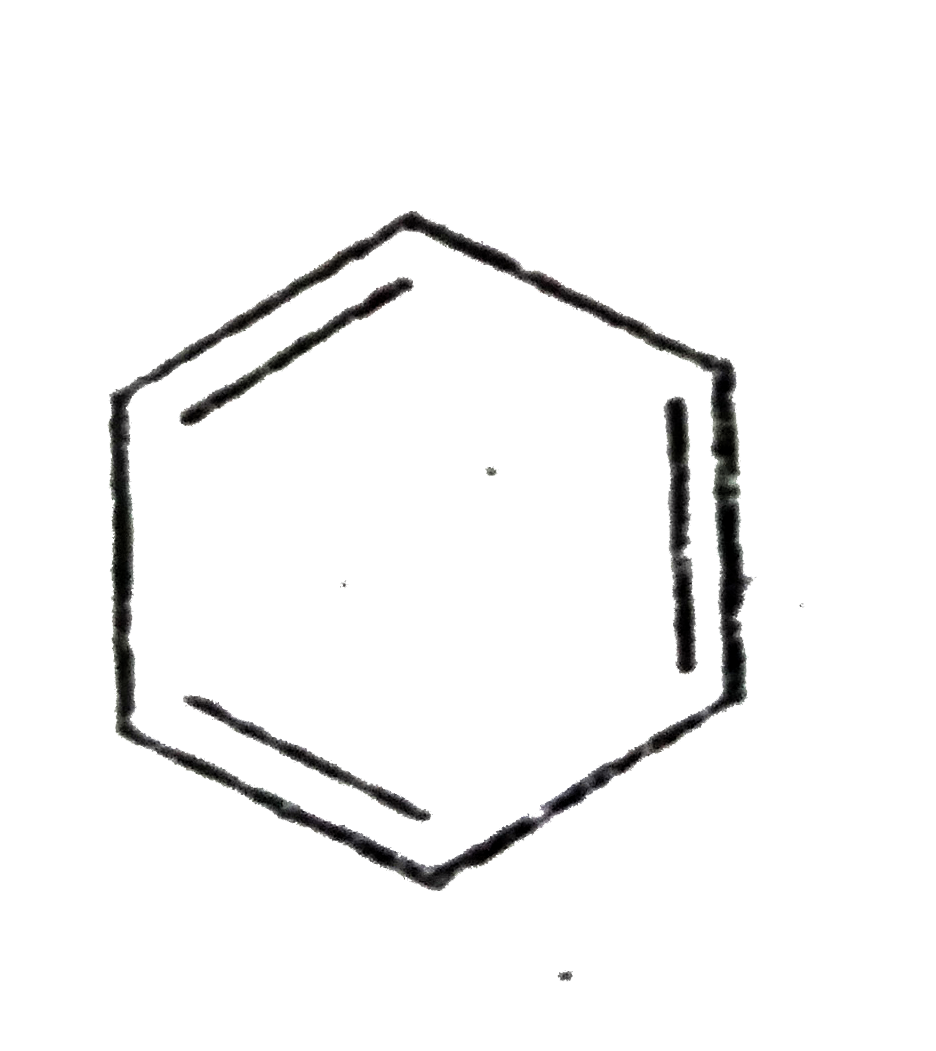
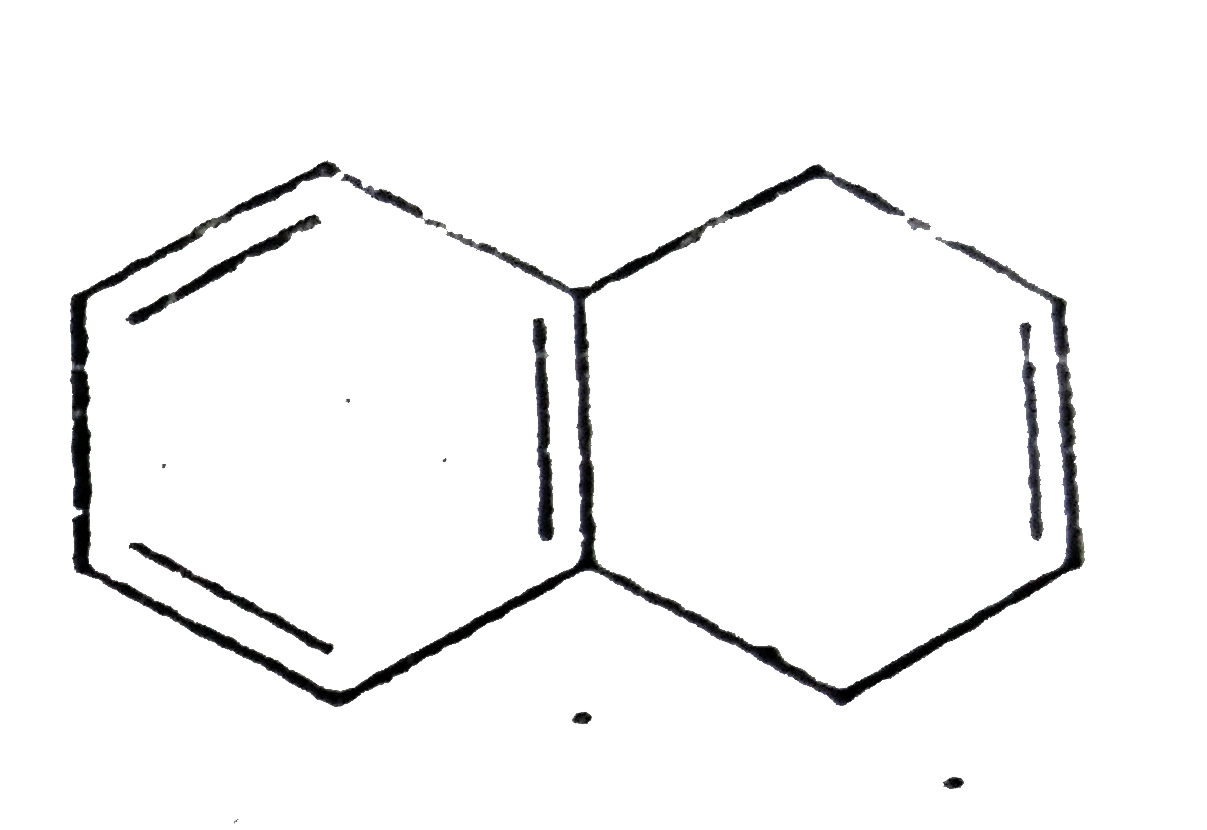
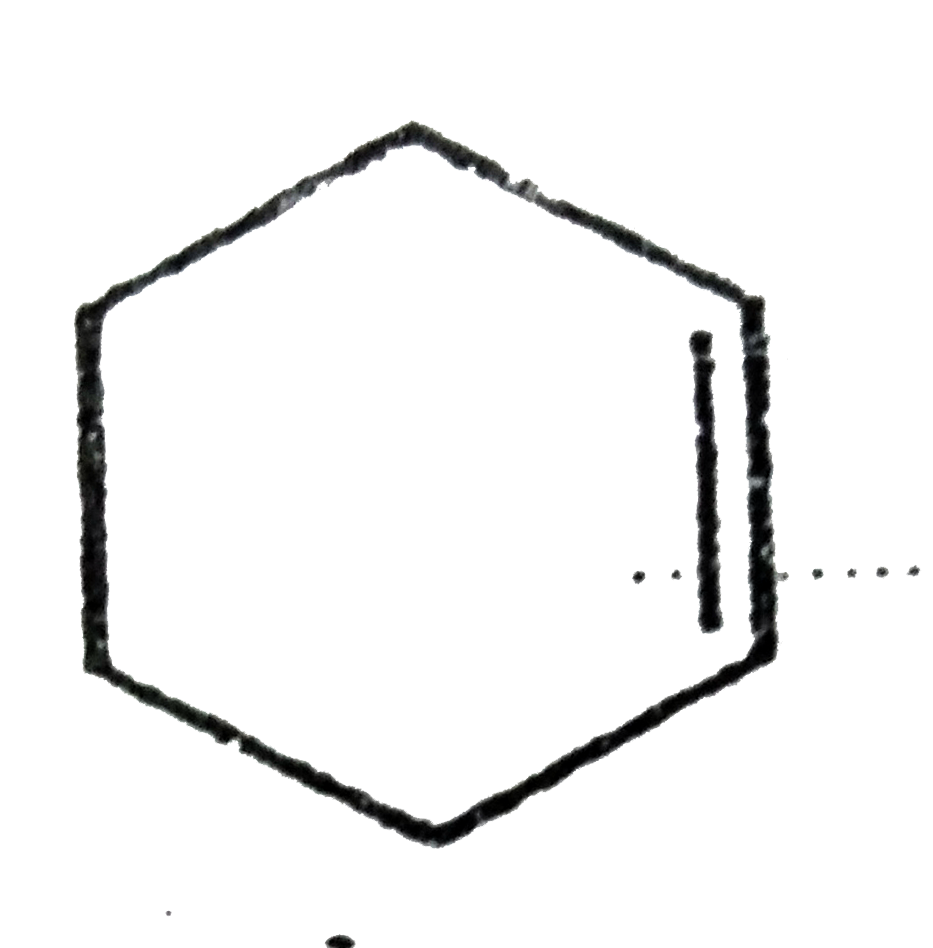
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
AAKASH INSTITUTE|Exercise SECTION-C OBJECTIVE TYPE QUESTIONS (MORE THAN ONE OPTIONS ARE CORRECT)|12 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise SECTION-D LINKED COMPREHENSION TYPE QUESTIONS|6 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION-A COMPETITION LEVEL DIFFER BY|50 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION -D|15 VideosHYDROGEN
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION - D) (Assertion-Reason Type question)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-HYDROCARBONS-SECTION-B OBJECTIVE TYPE QUESTIONS (ONE OPTION IS CORRECT)
- Consider the following reaction: CH(3)underset(D)underset(|)CH-under...
Text Solution
|
- In the given reaction CH(3)-overset(O)overset(||)(C)-C(2)H(5)overset...
Text Solution
|
- The most suitable sequence of reagents to perform this conversion is
Text Solution
|
- The compound having only primary hydrogen atoms is
Text Solution
|
- Which equation does not represent an example of Friedal-crafts reactio...
Text Solution
|
- In the reactions underset(CH2)overset(CH2)(||)underset"acid"overset"H...
Text Solution
|
- Arrange the following compounds in increasing order of reactivity towa...
Text Solution
|
- Among the following compounds, the decreasing order of reactivity towa...
Text Solution
|
- The alkene formed as a major product in the above elimination reaction...
Text Solution
|
- HBr reacts with CH(2)=CH-OCH(3) under anhydrous conditions at room tem...
Text Solution
|
- Colouration of Br(2)//C Cl(4) will be discharged by
Text Solution
|
- underset(Br)underset(|)(CH(2))-CH=CH-underset(Br)underset(|)(CH(2))ove...
Text Solution
|
- The ozonolysis product is
Text Solution
|
- Consider the following reaction The reactive intermediate invovled ...
Text Solution
|
- The intermediate during the addition of HCl to propene in the presence...
Text Solution
|
- Which of the following compound is most reactive towards an electrophi...
Text Solution
|
- The reaction is : CH(3)CHBr-CH(2)Br+2KOH("alc.")overset(Delta)rarr C...
Text Solution
|
- Which of the following alkene in acid catalysed hydration form 2-methy...
Text Solution
|
- The reaction of chlorine water with propene gives
Text Solution
|
- Point out A in the given reaction sequence Aoverset(O(3)//H(2)O(2))r...
Text Solution
|