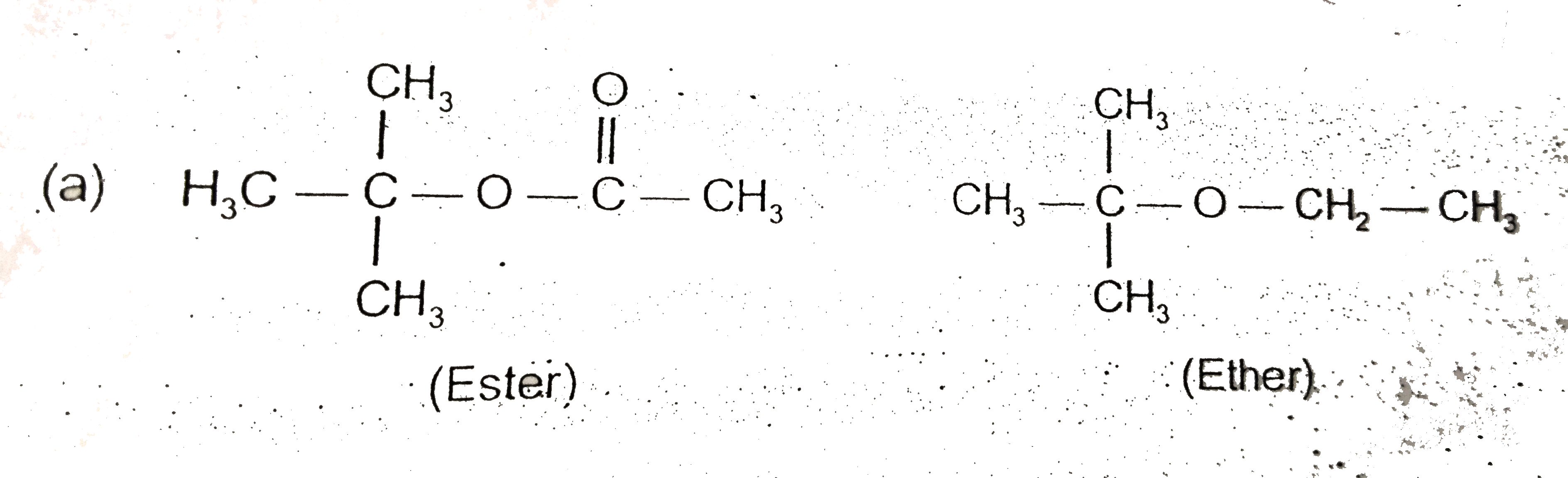
Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise Assignment (Section - I) (Aakash Challenger Questions)|5 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise Try Yourself|40 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise Assignment (Section - H) (Multiple True -False Type Questions)|2 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
AAKASH INSTITUTE|Exercise Try Yourself|33 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise Assignment(Section - D) (Assertion -Reason Type Question)|24 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-HALOALKANES AND HALOARENES -Assignment (Section - I) (Subjective Type Questions)
- Structure of one of the enantiomer of carvone is given below. Fin...
Text Solution
|
- Draw Fischer projection of Find the absolute configuration ...
Text Solution
|
- Give all the products expected including pertinent stereochemistr...
Text Solution
|
- Given the structure of the nucleophilic that could be used to ...
Text Solution
|
- Explain gives less substituted alkene as the major product ...
Text Solution
|
- Compare the nucleophilicity of given nucleophilies in aqueous medi...
Text Solution
|
- Tertiary alkyl halide undergoes solvolysis in either acetic aci...
Text Solution
|
- Both the reactants can be used to prepare styrene through dehyd...
Text Solution
|
- Two isomeric S(N)2 products are possible when sodium thiosulphat...
Text Solution
|
- Arrange the given species in the increasing order of Leaving ...
Text Solution
|