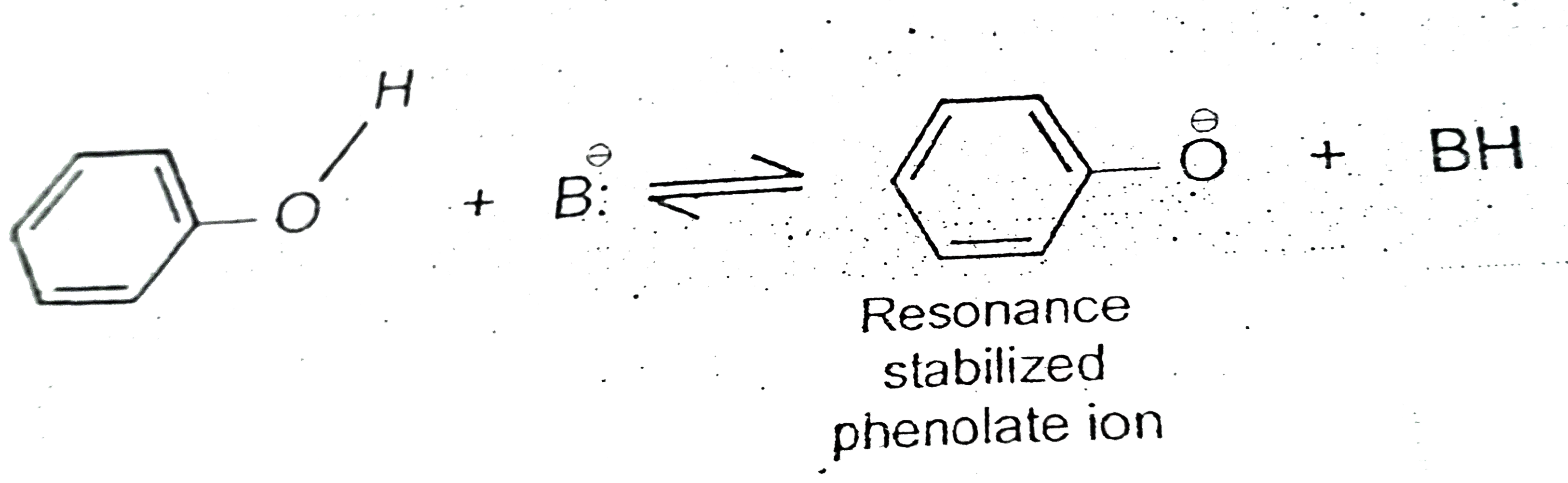
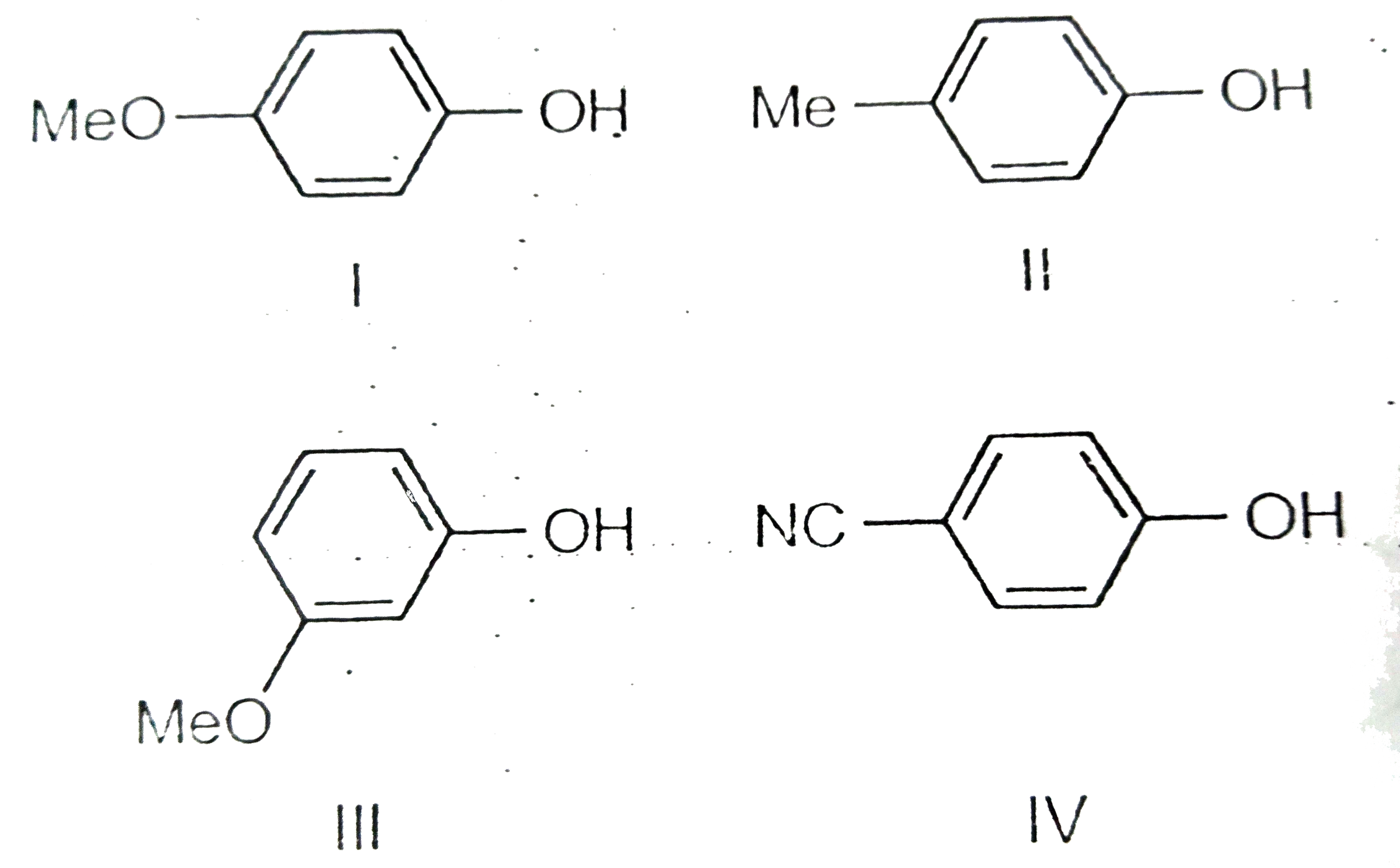
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Phenols are more acidic than aliphatic alcohols acidity of phen...
Text Solution
|
- Assertion : Phenols are more acidic than aliphatic alcohols. Reason ...
Text Solution
|
- Assertion (A) : Phenol is strongly acidic than ethanol. Reason (R) :...
Text Solution
|
- Phenols are more acidic than aliphatic alcohols acidity of phen...
Text Solution
|
- Phenols are more acidic than aliphatic alcohols acidity of phenols can...
Text Solution
|
- Phenols are more acidic than aliphatic alcohols because
Text Solution
|
- Assertion: The pK(a) of acetic acid is lower than that of phenol. R...
Text Solution
|
- Assertion: Phenol is more acidic than ethanol Reason: Phenoxide ion ...
Text Solution
|
- Assertion (A) : Phenol is more acidic than aliphatic alcohols. Reason ...
Text Solution
|