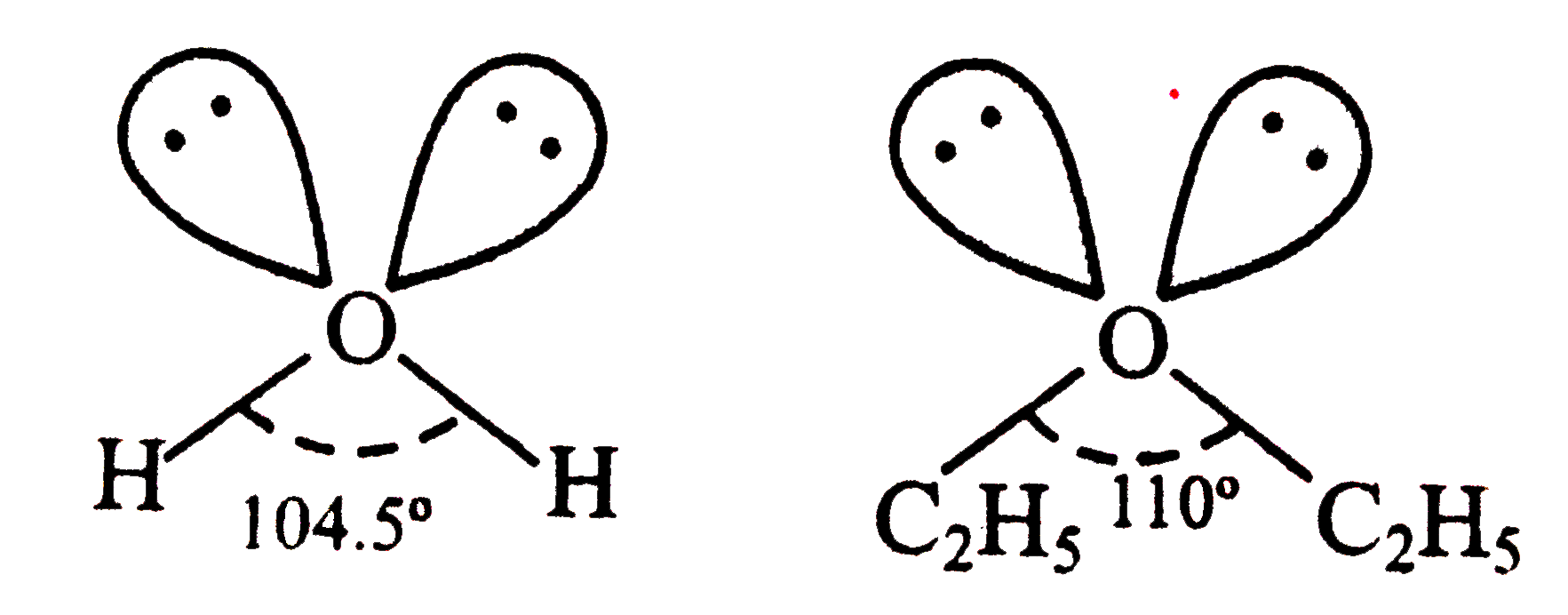Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)|3 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (I. MULTIPLE CHOICE QUESTIONS WITH ONE CORRECT ANSWER))|121 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (Questions)|1 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|9 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Assertion - Reason Type Question ) (Type II)|12 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CHEMICAL BONDING AND MOLECULAR STRUCTURE-ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS
- Arrange the following in order of (i) increasing N-O bond length (ii) ...
Text Solution
|
- Explain the shape of I(3)^(-) ion .
Text Solution
|
- Which of the following have identical bond order? (I) CN^(-) (II)O(...
Text Solution
|
- Arrange the following compounds in the icreasing order of bond length ...
Text Solution
|
- Indicate the type of bonds present in NH(4)NO(5) and state the mode of...
Text Solution
|
- Draw the Lewis structures of the species : CN^(-), I(3)^(-), C(3) O(2)...
Text Solution
|
- Why PCl(5) exists but NCl(5) does not ?
Text Solution
|
- Name and represent the type of bonds present in CuSO(4). 5H(2)O .
Text Solution
|
- In each of the following pairs of compounds, which one is more covalen...
Text Solution
|
- Give reason for the following : The molecule of MgCl(2) is linear ...
Text Solution
|
- Explain why bond angle of NH(3) is greater than NF(3) while bond angle...
Text Solution
|
- When 2s orbital overlaps with 2p(x) or 2p(y) orbital (assuming Z -axis...
Text Solution
|
- What would be the electronic configuration of HeH^(-) molecular ion ? ...
Text Solution
|
- H(2), Li(2) and B(2) all have the same bond order , viz, 1. Thun why ...
Text Solution
|
- Arrange the given dicarbon species in order of their bond lengths givi...
Text Solution
|
- Explain giving reasons whether BH(4)^(-) and H(3)O^(+) will have same ...
Text Solution
|
- In both water and diethyl ether, the central atom viz. O-atoms has sam...
Text Solution
|
- BCl(3) is planar but anhydrous AlCl(3) is tetrahedral . Explain .
Text Solution
|
- Calculate the percentage of p-character in the orbitals forming P-P ...
Text Solution
|
- Explain whether H^(+) ions will have greater mobility in ice or liquid...
Text Solution
|