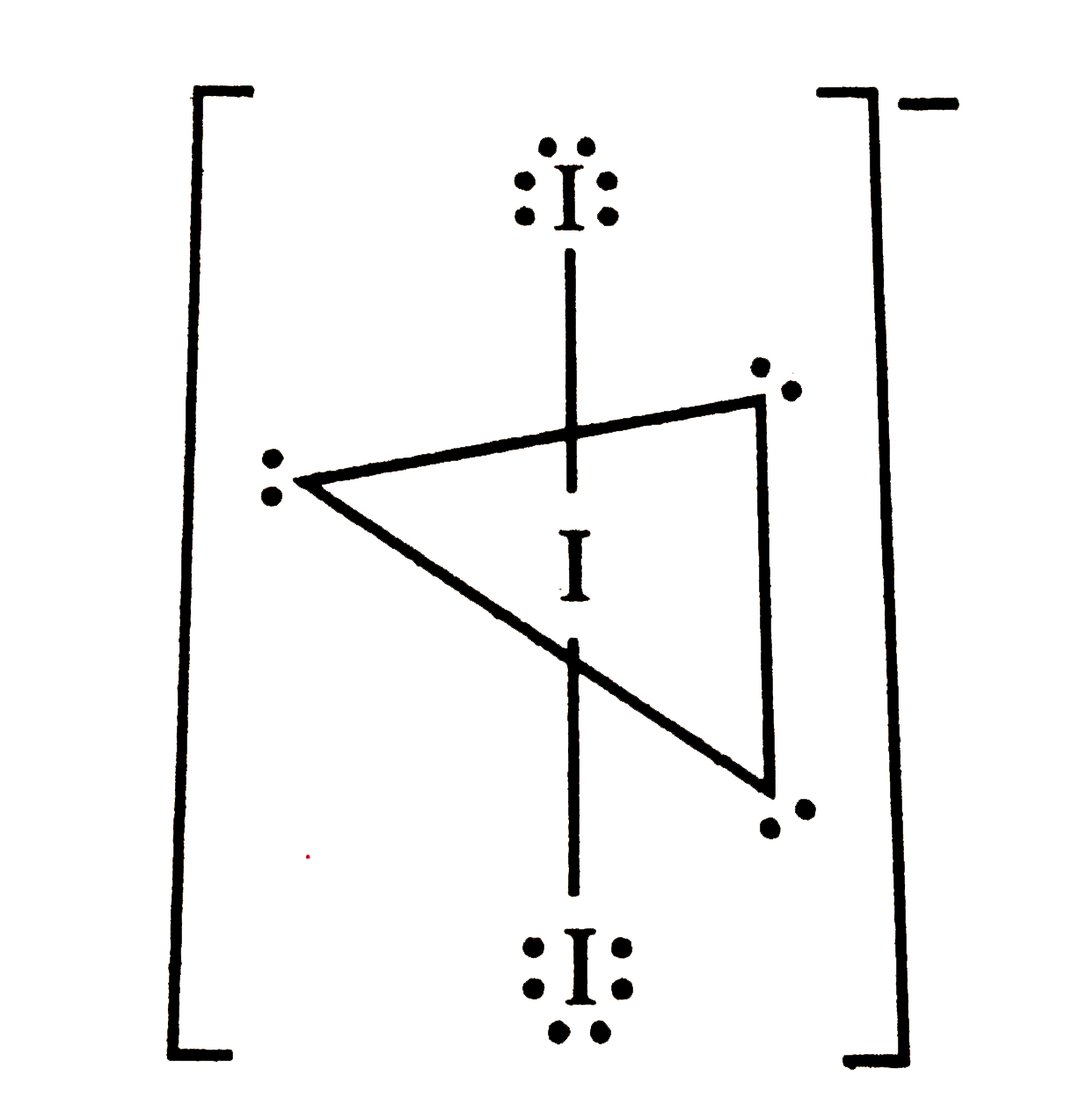
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (II. MULTIPLE CHOICE QUESTINS (WITH ONE OR MORE THAN ONE CORRECT ANSWER))|13 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (III. MULTIPLE CHOICE QUESTIONS (Based on the given Passage/Comprehension))|11 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)|3 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|9 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Assertion - Reason Type Question ) (Type II)|12 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CHEMICAL BONDING AND MOLECULAR STRUCTURE-COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (I. MULTIPLE CHOICE QUESTIONS WITH ONE CORRECT ANSWER))
- Which of the following has a regular geometry
Text Solution
|
- Predict the correct order of repulsions among the following :
Text Solution
|
- Total number of lone pair of electrons in 3 I(3)^(-) ion is
Text Solution
|
- The strength of the covalent bond in H(2) , F(2) and HF is in the o...
Text Solution
|
- The number and type of bonds between two carbon atoms in calcium carbi...
Text Solution
|
- How many bonds are there in
Text Solution
|
- In [Ag(CN)2]^(-), the number of pi bonds is
Text Solution
|
- Which of the following species contains equal number of pi and pi bond...
Text Solution
|
- The covalent bond length is the shortest in which of the following bon...
Text Solution
|
- v100subjectstringdiffnewFlow
Text Solution
|
- For AB bond if percent ionic character is plotted against electron...
Text Solution
|
- Arrange the following compounds in order of increasing dipole moment, ...
Text Solution
|
- Among the following, the molecule with the highest dipole moment is :
Text Solution
|
- Which one of the following arrangements of molecules is correct on the...
Text Solution
|
- Among the following, the molecule with the highest dipole moment is :
Text Solution
|
- Which of the following has maximum dipole moment?
Text Solution
|
- Which are non-polar molecules? I. NCl(3) " "II. SO(3)" ...
Text Solution
|
- Which bond angle theta would result in the maximum dipole moment for...
Text Solution
|
- The dipole moment of The dipole moment of
Text Solution
|
- The correct order of increasing polarising power of the cations in the...
Text Solution
|