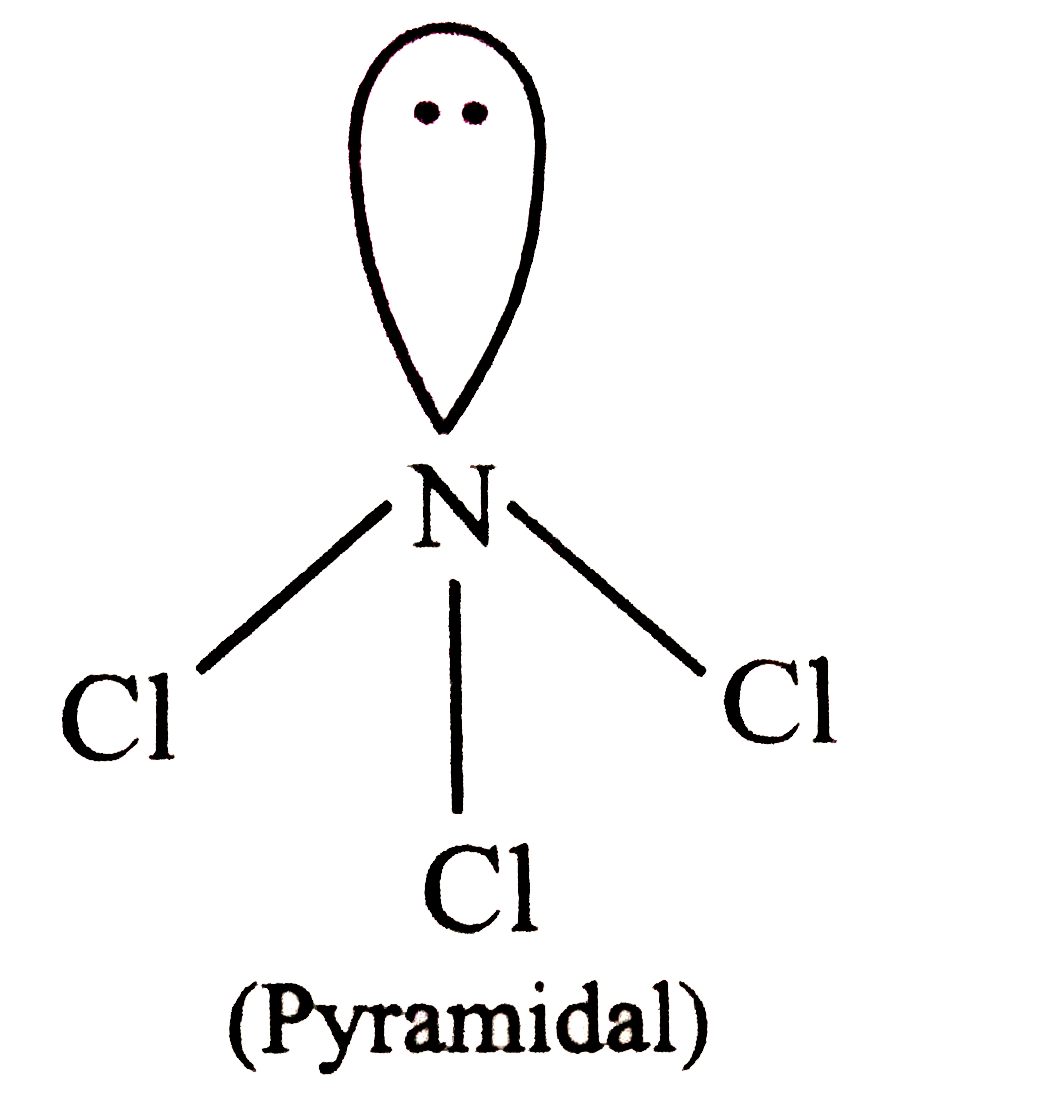
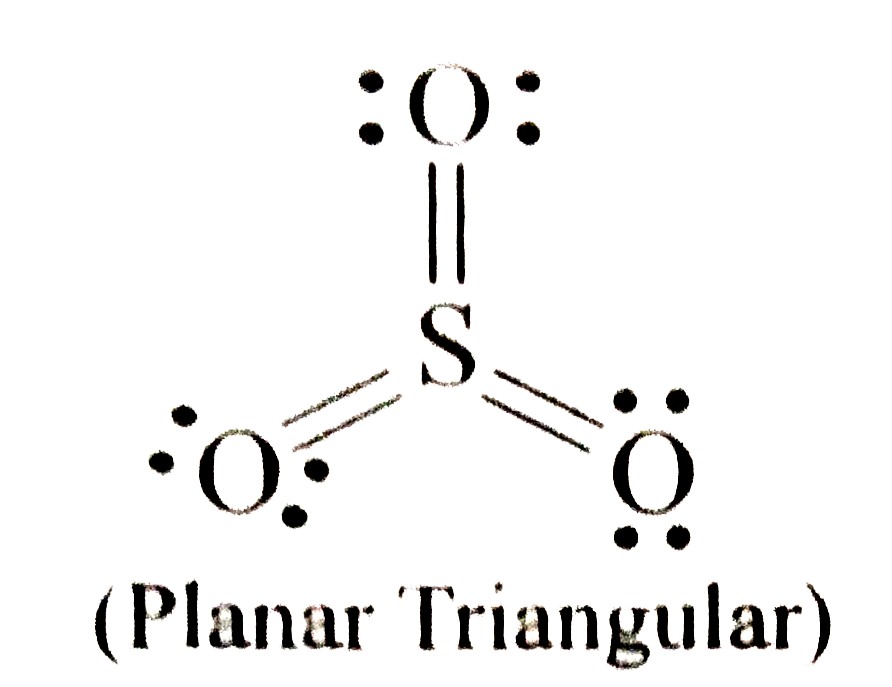
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (II. MULTIPLE CHOICE QUESTINS (WITH ONE OR MORE THAN ONE CORRECT ANSWER))|13 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (III. MULTIPLE CHOICE QUESTIONS (Based on the given Passage/Comprehension))|11 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)|3 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|9 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Assertion - Reason Type Question ) (Type II)|12 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CHEMICAL BONDING AND MOLECULAR STRUCTURE-COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (I. MULTIPLE CHOICE QUESTIONS WITH ONE CORRECT ANSWER))
- Among the following, the molecule with the highest dipole moment is :
Text Solution
|
- Which of the following has maximum dipole moment?
Text Solution
|
- Which are non-polar molecules? I. NCl(3) " "II. SO(3)" ...
Text Solution
|
- Which bond angle theta would result in the maximum dipole moment for...
Text Solution
|
- The dipole moment of The dipole moment of
Text Solution
|
- The correct order of increasing polarising power of the cations in the...
Text Solution
|
- The charge/size ratio of a cation determines its polarizing power. Whi...
Text Solution
|
- Which of the following is a polar molecule ?
Text Solution
|
- For which of the following molecules, significant mu ne o ?
Text Solution
|
- Some ether is added to anb aqueous soluetion of a mixtrue of LiCl, N...
Text Solution
|
- Among the following species, identify the isostuctural pairs NF(3). ...
Text Solution
|
- Which of the following pairs of ions are isoelectronic and isostructur...
Text Solution
|
- The type of hybrid orbitals used by chlorine atom in ClO(3)^(-) is
Text Solution
|
- Which one of the following compounds has sp^(2) hybridisation ? .
Text Solution
|
- The hybridization of atomic orbitals of nitrogen is NO(2)^(+), NO(3)^(...
Text Solution
|
- The correct order of hybridisation of the central atom in the followin...
Text Solution
|
- The shapes of SF(4)andXeF(2) respectively are
Text Solution
|
- The pair having similar geometry is
Text Solution
|
- The maximum number of 90^(@) angles between bond pair-bond pair of ele...
Text Solution
|
- The ion with maximum number of lone pairs on the central atom is-
Text Solution
|