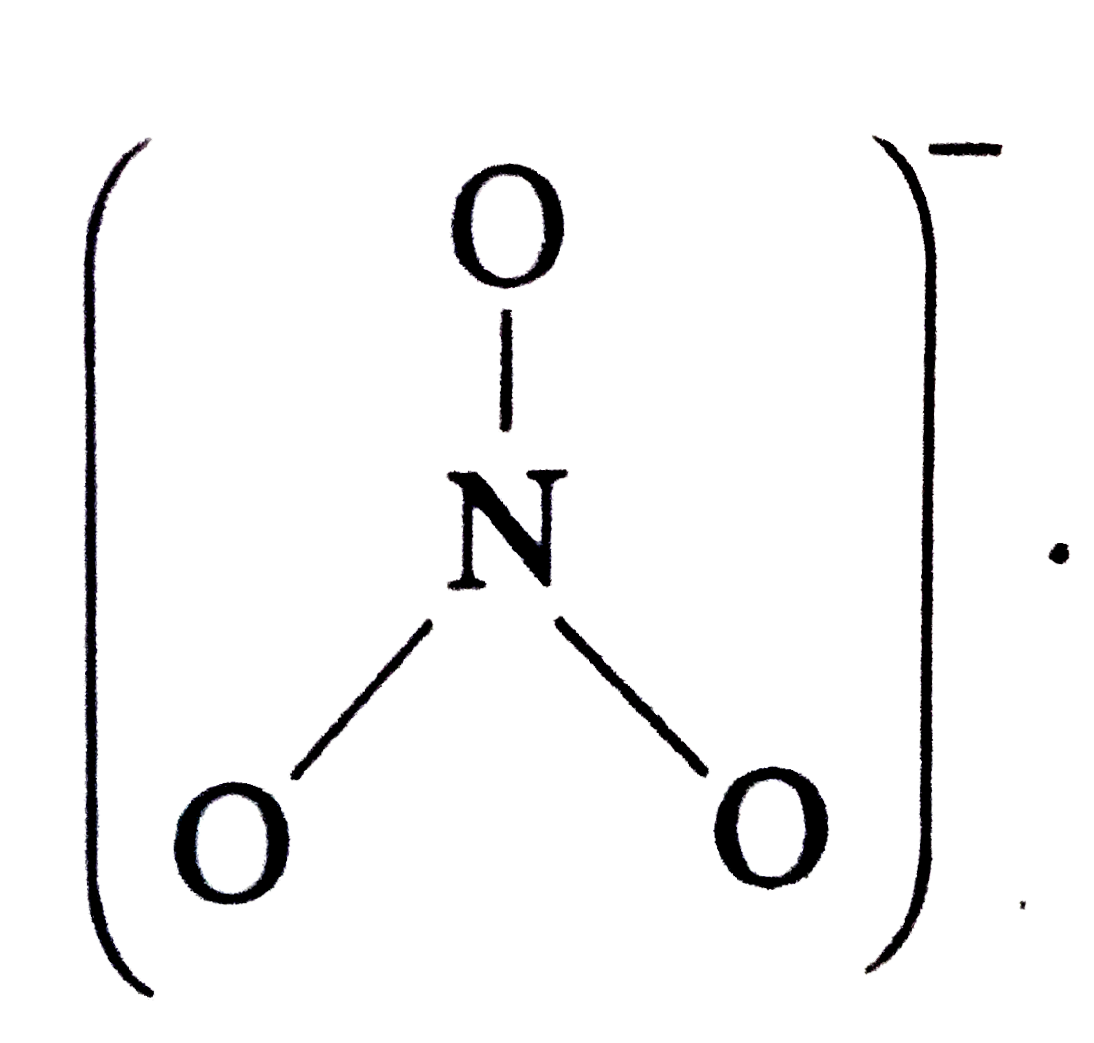
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (II. MULTIPLE CHOICE QUESTINS (WITH ONE OR MORE THAN ONE CORRECT ANSWER))|13 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (III. MULTIPLE CHOICE QUESTIONS (Based on the given Passage/Comprehension))|11 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)|3 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|9 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Assertion - Reason Type Question ) (Type II)|12 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CHEMICAL BONDING AND MOLECULAR STRUCTURE-COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (I. MULTIPLE CHOICE QUESTIONS WITH ONE CORRECT ANSWER))
- The nodal plane in the pi-bond of ethene is located in:
Text Solution
|
- Shape of O(2)F(2) is similar to that of
Text Solution
|
- The ONO bond angle is maximum in
Text Solution
|
- In I(3)^(-) , Lewis base is
Text Solution
|
- In which of the following molecules are all the bonds not equal ?
Text Solution
|
- Which of the following species has a linear shape ?
Text Solution
|
- If I2 is dissolved in aqueous KI, the intense yellow species I3^ is fo...
Text Solution
|
- In which pair of species, both species do have similar geometry
Text Solution
|
- The incorrectly matched pair, among the following is
Text Solution
|
- Two types of FXF angles are present in which of the following molecule...
Text Solution
|
- Out of N(2) O , SO(2), I(3)^(+), I(3)^(-), H(2)O, NO(2)^(-) and N(3)^...
Text Solution
|
- Which of the following species is non-linear ?
Text Solution
|
- The species having pyramidal shape is
Text Solution
|
- The correct order of increasing bond angles in the following species i...
Text Solution
|
- Among the following molecules : SO(2),SF(4) ,CIF(3) ,BrF(5) , and XeF(...
Text Solution
|
- XeF(2) is isostructural with
Text Solution
|
- The species in which the N-atom is in a state of sp hybridisation is
Text Solution
|
- Consider the molecules CH(4),NH(3) and H(2)O which of the given statem...
Text Solution
|
- The species having bond angle of 120^(@) is
Text Solution
|
- Which of the following pairs of compound is isoelectronic and isostruc...
Text Solution
|