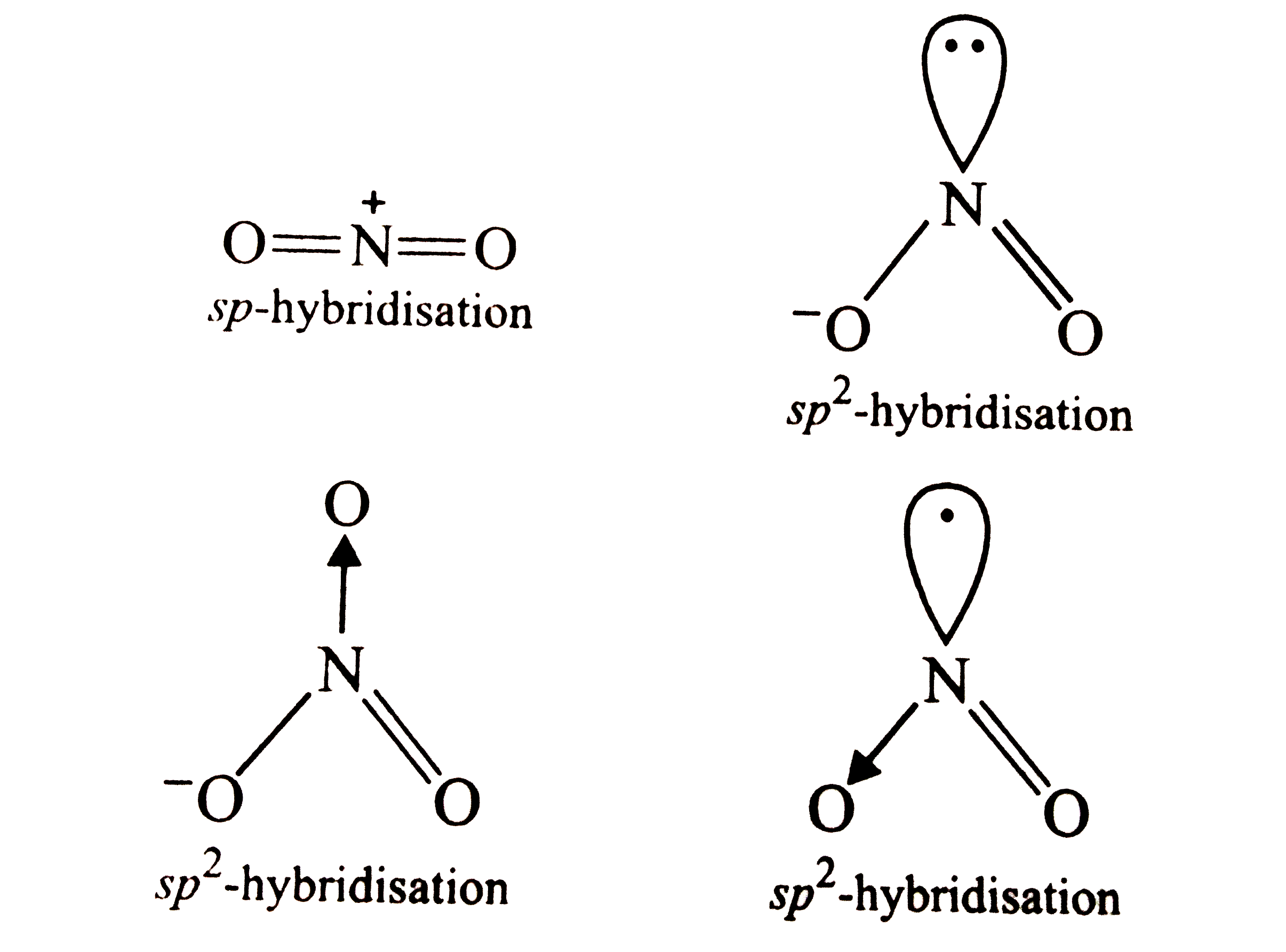A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (II. MULTIPLE CHOICE QUESTINS (WITH ONE OR MORE THAN ONE CORRECT ANSWER))|13 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (III. MULTIPLE CHOICE QUESTIONS (Based on the given Passage/Comprehension))|11 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)|3 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|9 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Assertion - Reason Type Question ) (Type II)|12 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CHEMICAL BONDING AND MOLECULAR STRUCTURE-COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (I. MULTIPLE CHOICE QUESTIONS WITH ONE CORRECT ANSWER))
- Among the following molecules : SO(2),SF(4) ,CIF(3) ,BrF(5) , and XeF(...
Text Solution
|
- XeF(2) is isostructural with
Text Solution
|
- The species in which the N-atom is in a state of sp hybridisation is
Text Solution
|
- Consider the molecules CH(4),NH(3) and H(2)O which of the given statem...
Text Solution
|
- The species having bond angle of 120^(@) is
Text Solution
|
- Which of the following pairs of compound is isoelectronic and isostruc...
Text Solution
|
- Which one of the following contains ionic , covalent and coordinate bo...
Text Solution
|
- Which of the following has p pi - d pi bonding ?
Text Solution
|
- The correct stability order of the following resonance structures is ...
Text Solution
|
- Which of the following is a the most preferred and hence of the lower ...
Text Solution
|
- Consider the statements : I . Bond length in N(2)^(+) is 0.002 Å g...
Text Solution
|
- The correct order of increasig C-O bond length of CO, CO(3)^(2-), CO(2...
Text Solution
|
- In which of the following ionixation processes , the bond order has in...
Text Solution
|
- The species having bond order different from that in CO is
Text Solution
|
- The correct order of bond order values among the following (i) NO^(...
Text Solution
|
- Which one of the following pairs consists of only paramagnetic species
Text Solution
|
- The magnetic moment of KO(2) at room temperature is ---------- BM.
Text Solution
|
- Which of the following options represents the correct bond order ?
Text Solution
|
- Decreasing order of stability of O(2), O(2)^(-), O(2)^(+) and O(2)^(2-...
Text Solution
|
- Four diatomic species are listed below in different sequences. Which o...
Text Solution
|