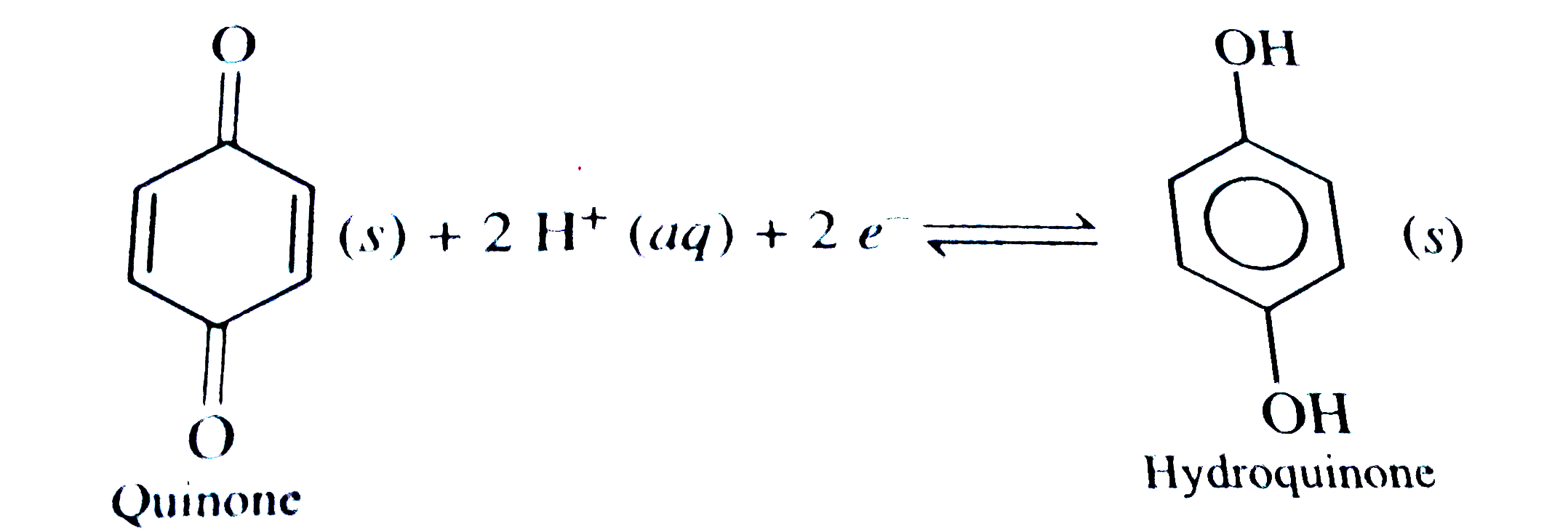A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
PRADEEP|Exercise VALUE BASED QUESTIONS WITH ANWER (Multiple Choice Questions-II)|7 VideosELECTROCHEMISTRY
PRADEEP|Exercise VALUE BASED QUESTIONS WITH ANWER (Multiple Choice Questions-III Based on the given Passage/Comprehension)|13 VideosELECTROCHEMISTRY
PRADEEP|Exercise HIGHER ORDER THINKING SKILLS (HOTS PROBLEMS)|13 VideosD- AND F-BLOCK ELEMENTS
PRADEEP|Exercise IMPORTANT QUESTIONS|30 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
PRADEEP|Exercise Curiosity Questions|2 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ELECTROCHEMISTRY-VALUE BASED QUESTIONS WITH ANWER (Multiple Choice Questions-I)
- When measured against a standard calomel electrode, an electrode is fo...
Text Solution
|
- Which has maximum potential for the half-cell reaction : 2H^(+)2e^(-) ...
Text Solution
|
- Quinhydrone electrode is sometimes used to find the pH of a solution. ...
Text Solution
|
- Given E(Cr^(3+)//Cr^(@))= -O*74V,E(MnO(4)^(-)//Mn^(2+))^(@) = 1.51V E...
Text Solution
|
- Given E(Cl(2)//Cl^(-))^(@)=1.36V,E(Cr^(3+)//Cr)^(@)=-0.74V E("Cr(2)O...
Text Solution
|
- Standard reduction potentials of the half reactions are given below ...
Text Solution
|
- Small quantities of compounds TX, TY and TZ are put into separate test...
Text Solution
|
- Which of the following statements are correct concerning redox propert...
Text Solution
|
- The standard reduction potentials for Zn^(2+)//Zn,Ni^(2+)//Niand Fe^(2...
Text Solution
|
- Standard electrode potential for Sn^(4+)//Sn^(2+) couple is +0.15 V an...
Text Solution
|
- A button cell used in watched funcations as follwing Zn(s)+Ag(2)O(s)...
Text Solution
|
- A solution contains Fe^(2+), Fe^(3+) and T^(-) ions. This solution was...
Text Solution
|
- Given that E(Fe^(2+)//Fe)^(.)=-0.44V,E(Fe^(3+)//Fe^(2+))^(@)=0.77V if...
Text Solution
|
- Zn gives H(2) gas with H(2)SO(4) and HCl but not with HNO(3) because
Text Solution
|
- Cr(2)O(7)^(2-)+I^(-)toI(2)+Cr^(3+) E(cell)^(@)=0.79V,E(Cr(2)O(7)^(2-...
Text Solution
|
- Aluminium displaces hydrogen from dilute HCl whereas silver does not. ...
Text Solution
|
- An aqueous solution containing one mole per litre of each Cu(NO(3))(2...
Text Solution
|
- The EMF of a cell formed by combining a particular electrode with stan...
Text Solution
|
- Given below are the half -cell reactions Mn^(2+)+2e^(-) to Mn, E^(@)...
Text Solution
|
- A hydrogen gas electrode is made by dipping platinum wire in a solutio...
Text Solution
|