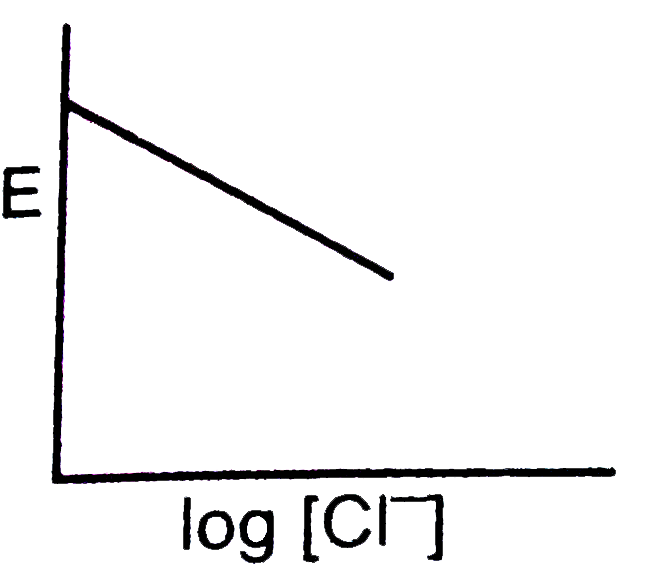
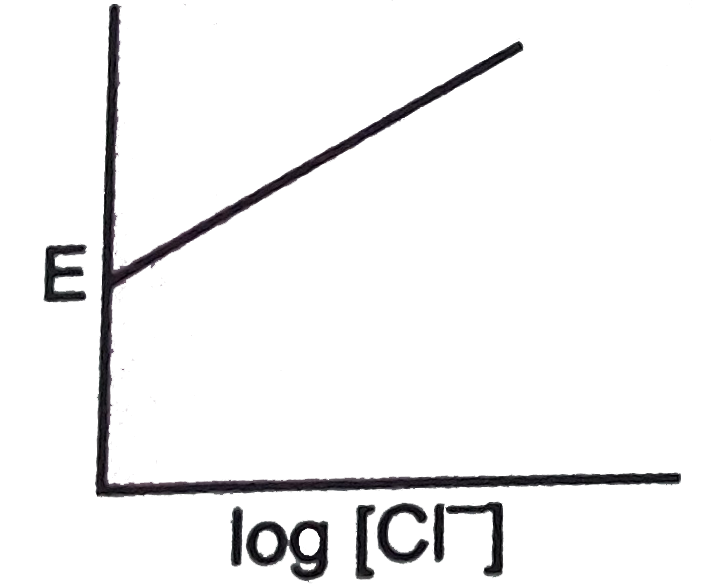
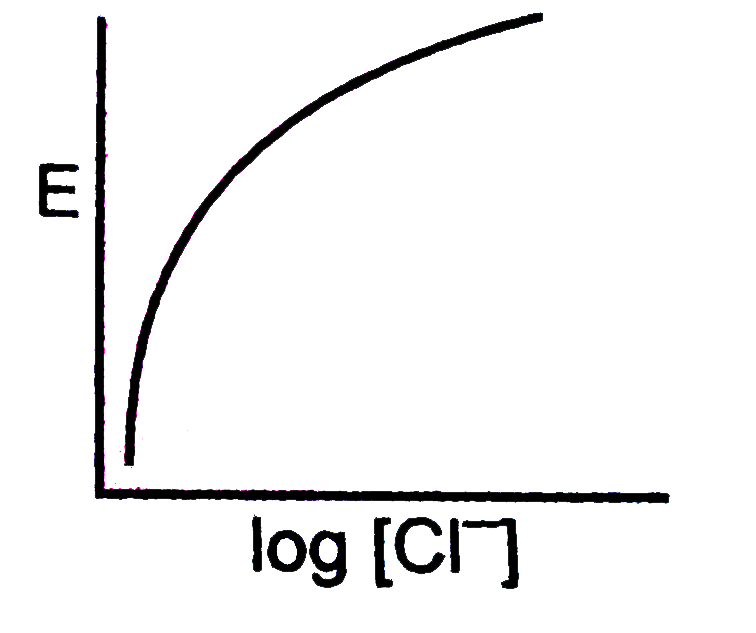
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
PRADEEP|Exercise VALUE BASED QUESTIONS WITH ANWER (Multiple Choice Questions-II)|7 VideosELECTROCHEMISTRY
PRADEEP|Exercise VALUE BASED QUESTIONS WITH ANWER (Multiple Choice Questions-III Based on the given Passage/Comprehension)|13 VideosELECTROCHEMISTRY
PRADEEP|Exercise HIGHER ORDER THINKING SKILLS (HOTS PROBLEMS)|13 VideosD- AND F-BLOCK ELEMENTS
PRADEEP|Exercise IMPORTANT QUESTIONS|30 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
PRADEEP|Exercise Curiosity Questions|2 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ELECTROCHEMISTRY-VALUE BASED QUESTIONS WITH ANWER (Multiple Choice Questions-I)
- In a cell that utilizes the reactions. Zn(s) + 2H^(+) (aq) rightarro...
Text Solution
|
- For the electrode Cu//Cu^(2+),log[Cu^(2+)] (along X-axis) is plotted a...
Text Solution
|
- For the calomel half-cell, Hg, Hg(2)Cl(2)|Cl^(-)(aq) values of electro...
Text Solution
|
- The following cell is found to have EMF equal to zero. Pt,H(2)("x at...
Text Solution
|
- The emf of the cell, Zn|Zn^(2+)(0.01M)|| Fe^(2+)(0.001M) | Fe at 2...
Text Solution
|
- Find K(c) for the complex: [Ag(NH(3))(2)]^(o+)hArrAg^(o+)+2NH(3) E...
Text Solution
|
- Given : Hg(2)^(2+) rightarrow 2Hg, E^(@) = 0.789 V and Hg^(2+) + 2e^...
Text Solution
|
- For the following electrochemical cell at 298K Pt(s)+H(2)(g,1"bar") ...
Text Solution
|
- The emf of a Daniell cell at 298 K is E(1) Zn|ZnSO(4)(0.01 M)||CuSO(...
Text Solution
|
- For the following cell, Zn(s)|ZnSO(4)(aq)||CuSO(4)(aq)||Cu(s) When...
Text Solution
|
- Standard free energies of formation (I kJ //mol ) at 298 K are -237 ....
Text Solution
|
- A fuel cell involves combustion of butane at at 1 atm and 298 K unders...
Text Solution
|
- If the E(cell)^(@) for a given reaction has a positive value, then whi...
Text Solution
|
- The half cell reaction for rusting of iron are: 2H^(+)+2e^(-)+(1)/(2...
Text Solution
|
- In the electrolysis of which solution OH^(-) ions are discharged in p...
Text Solution
|
- Which pair of electrolytes could not be distinguished by the products ...
Text Solution
|
- The metal that cannot obtained by electrolysis of an aqueous solution ...
Text Solution
|
- In the lead-acid battery during charging, the cathode reaction is
Text Solution
|
- In a fuel cell methanol is used as fuel and oxygen gas is used as an o...
Text Solution
|
- Rust is a mixture of
Text Solution
|