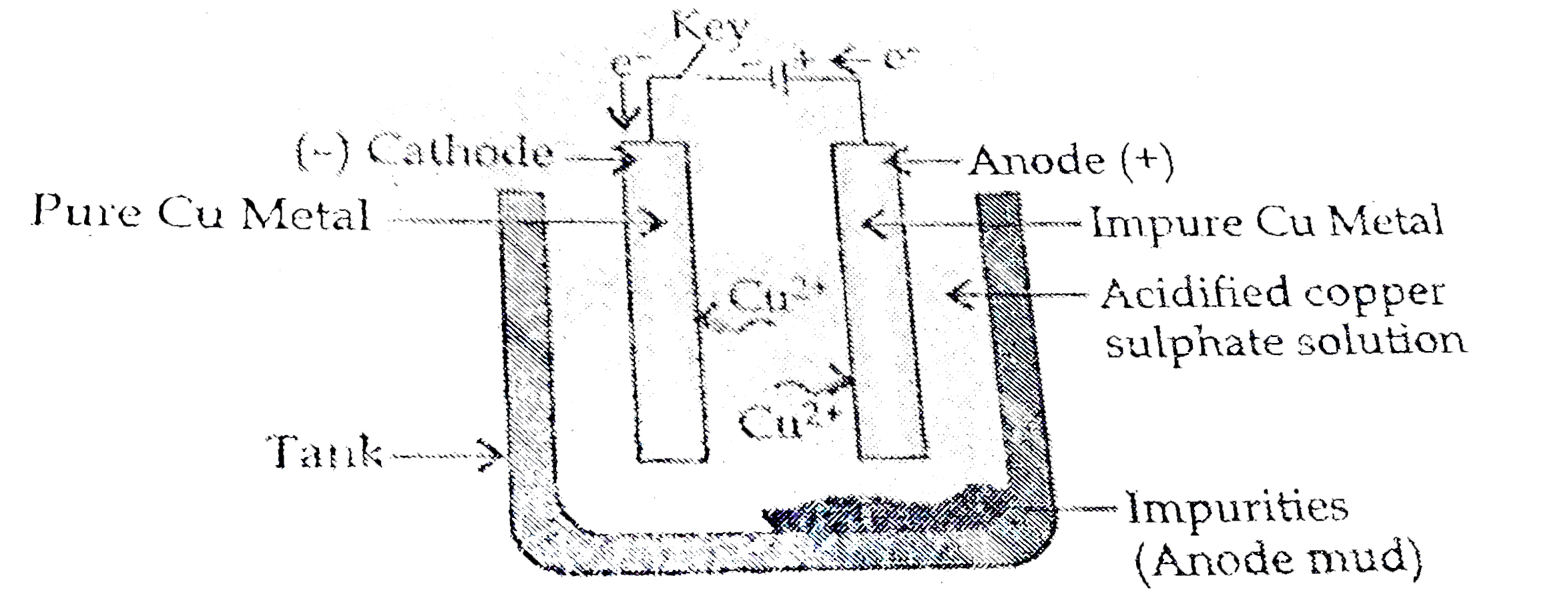Some of the moderately reactive metals occur in nature as their carbonates. It is easier to obtain metals from their oxides (by reduction) than from carbonates.
So before reduction the carbonte ore must be converted into metal oxide by the process of calcination.
Calcination is the process in which a carbonate ore is heated strongly in the absence of air to convert it into metal oxide.
`underset(("Calamine ore"))underset("Zinc carbonate")(ZnCO_(3)) overset("Calcination")rarr underset("Zinc oxide")(ZnO(s)) + CO_(2)(g)`
The metal oxide obtained by calcination is converted to free metal by using reducing agents like carbon, aluminium sodium or calcium . The reducing agent used depends on the chemical reactivity of the metal to be extracted .
`underset("Zinc oxide")(ZnO) + C(s) rarr underset("Zinc metal")(Zn(s)) + CO(g)`
Iron metal is extracted from its oxides by reduction with carbon. Tin and lead metals are also extracted by the reduction of their oxides with carbon.
(b ) Copper can be extracted by heating its sulphide ore in the presence of excess of air.
This process is called roasting.
Steps in the roasting of copper,
(i) The concentrated copper (I) sulphide ore is heated in air till a part of copper (I) sulphide is oxidised to copper (I) oxide.
`underset("Copper glance ore")(2Cu_(2)S(s)) + 3O_(2)(g) overset("Roasted")(rarr) underset("Copper oxide")(2Cu_(2)O(s)) + 2SO_(2)(s)`
(ii) When a good amount of copper (I) sulphide has been converted into copper (I) oxide, then the supply of air for roasting is stopped. In the absence of air, copper (I) oxide reacts with the remaining copper (I) sulphide to form copper metal and sulphur dioxide.
`2Cu_(2)O(s) + + Cu_(2)S(s) overset("Heat")rarr underset("Copper metal")(6Cu(s)) + SO_(2)(g)`
(iii) The obtaine metal is then purified by electrolytic refining.
Electrolytic refining of copper ,