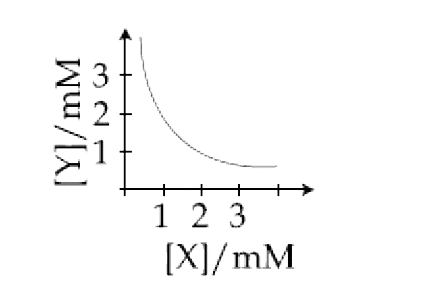A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAIN-CHEMISTRY
- Reagent used for the given conversion is :
Text Solution
|
- Among the gases (a)-(e) the gases that cause greenhouse eFIGUREFIGUREe...
Text Solution
|
- The stoichiometre and solubility product oFIGURE a salt with the solub...
Text Solution
|
- ' A. Intermolecular force of attraction of X gt Y B. Intermolecula...
Text Solution
|
- Two liquids isohexane and 3-methylpentane has boiling point 60^@C and ...
Text Solution
|
- The complex that can show FIGUREac- and mer-isomers is
Text Solution
|
- A gas undergoes expansion according to the following graph. Calculate ...
Text Solution
|
- Given 2H(2)O rarr O2 + 4H^+ + 4e^- , E0 = -1.23 V. Calculate electrode...
Text Solution
|
- Calculated the mass of FeSO(4).7H(2)O, which must be added in 100 kg o...
Text Solution
|
- Number of chiral centres in Pencillin is
Text Solution
|
- 0.3 g [ML(6)]Cl(3) of molar mass 267.46 g/mol is reacted with 0.125 M ...
Text Solution
|
- Preparation of Bakelite proceeds via reactions:
Text Solution
|
- The major product in the following reaction is :
Text Solution
|
- White phosphorus on reaction with concentrated NaOH solution in an ine...
Text Solution
|
- Kjeldahl method cannot be used for :
Text Solution
|
- Which of the following compounds is likely to show both Frenkel a...
Text Solution
|
- Arrange the following bonds according to their averge bond energies ...
Text Solution
|
- Consider the following plots of rate constant versus (1)/(T) f...
Text Solution
|
- Assertion: pH of water increases on increasing temperature. Reason: ...
Text Solution
|
- Hydrogen has three isotopes (A),(B) and (C). If the number of neu...
Text Solution
|