Text Solution
Verified by Experts
Topper's Solved these Questions
MISCELLANEOUS QUESTIONS
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Effects of Electric Current|8 VideosMISCELLANEOUS QUESTIONS
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Heat|7 VideosMISCELLANEOUS QUESTIONS
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Periodic Classification of Elements|12 VideosMATCH THE COLUMNS
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise All Questions|36 VideosMODEL ACTIVITY SHEET
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise ANSWER THE FOLLOWING|5 Videos
Similar Questions
Explore conceptually related problems
NAVNEET PUBLICATION - MAHARASHTRA BOARD-MISCELLANEOUS QUESTIONS -Chemical Reactions and Equations
- Explain the term reactant and product giving examples.
Text Solution
|
- Write the balanced equations for the following reactions : (i) H(2...
Text Solution
|
- (ii) Ag(s) +HCI(I) to AgCI darr + H(2) uarr
Text Solution
|
- (iii) H(2)SO(4(aq)) + NaOH((aq)) to Na(2)SO(4(aq)) + H(2)O((I))
Text Solution
|
- (a) How does the rate of reaction depend upon the concentration of rea...
Text Solution
|
- (a) How does the rate of reaction depend upon the concentration of rea...
Text Solution
|
- How does the rate of a reaction depend upon the temperature of reactan...
Text Solution
|
- How does the rate of a reaction depend upon the catalyst ? Give a sui...
Text Solution
|
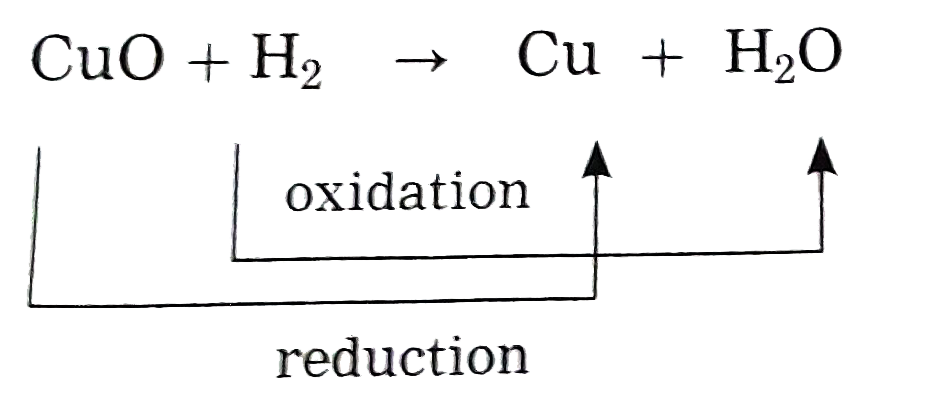
- Identify the substances that are oxidised and the substances that are ...
Text Solution
|
- Identify the substances that are oxidised and the substances that are ...
Text Solution
|
- Identify the substances that are oxidised and the substances that are ...
Text Solution
|
- Identify the following reactions the reactants that undergo oxidation ...
Text Solution
|
- 2Ag(2)O to 4Ag + O(2) uarr
Text Solution
|
- 2Mg + O(2) to 2MgO
Text Solution
|
- Identify from the following reactions the reactants that undergo oxida...
Text Solution
|
- Observe the following picture and write down the chemical reaction wi...
Text Solution
|
- Complete the process of iron rusting by filling the blanks. Suggest a ...
Text Solution
|
- RANCIDITY
Text Solution
|