Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUNIL BATRA (41 YEARS IITJEE PHYSICS)-HEAT AND THERMODYNAMICS-JEE Main And Advanced
- A thin tube of uniform cross section is sealed at both ends. It lies h...
Text Solution
|
- An ideal gas has a specific heat at constant pressure CP=(5R)/2. The g...
Text Solution
|
- Two moles of helium gas(lambda=5//3) are initially at temperature 27^@...
Text Solution
|
- An ideal monatomic gas is confined in a cylinder by a spring-loaded pi...
Text Solution
|
- An ideal gas having initial pressure P, volume V and temperature T is ...
Text Solution
|
- Three moles of an ideal gas (Cp=7/2R) at pressure, PA and temperature ...
Text Solution
|
- Two moles of helium gas undergo a cyclic process as shown in Fig. Assu...
Text Solution
|
- One mole of a monoatomic ideal gas is taken through the cycle shown in...
Text Solution
|
- An ideal gas is taken through a cyclic thermodynamic process through f...
Text Solution
|
- A closed container of volume 0.02m^3contains a mixture of neon and arg...
Text Solution
|
- A gaseous mixture enclosed in a vessel of volume V consists of one mol...
Text Solution
|
- At 27^@C two moles of an ideal monoatomic gas occupy a volume V. The g...
Text Solution
|
- The temperature of 100g of water is to be raised from 24^@C to 90^@C b...
Text Solution
|
- One mole of a diatomic ideal gas (gamma=1.4) is taken through a cyclic...
Text Solution
|
- The apparatus shown in the figure consists of four glass columns conn...
Text Solution
|
- One mole of an ideal monatomic gas is taken round the cyclic process A...
Text Solution
|
- A solid body X of heat capacity C is kept in an atmosphere whose tempe...
Text Solution
|
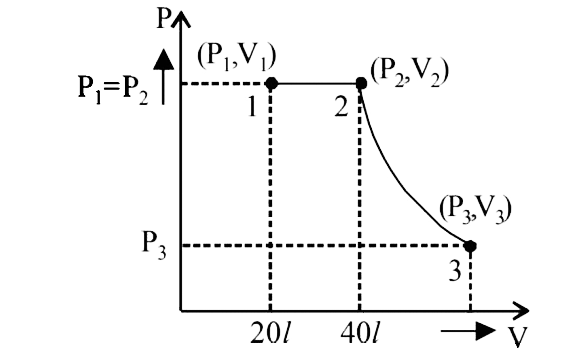
- Two moles of an ideal monatomic gas, initially at pressure p1 and volu...
Text Solution
|
- Two moles of an ideal monatomic gas is taken through a cycle ABCA as s...
Text Solution
|
- An ice cube of mass 0.1kg at 0^@C is placed in an isolated container w...
Text Solution
|