Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUNIL BATRA (41 YEARS IITJEE PHYSICS)-HEAT AND THERMODYNAMICS-JEE Main And Advanced
- A diatomic ideal gas is compressed adaibatically to 1/32 of its initia...
Text Solution
|
- Steel wire of length 'L' at 40^@C is suspended from the ceiling and th...
Text Solution
|
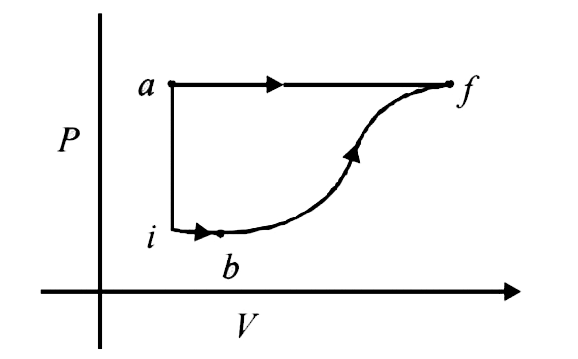
- A thermodynamic system is taken from an initial state I with internal ...
Text Solution
|
- Two spherical stars A and B emit blackbody radiation. The radius of A ...
Text Solution
|
- A metal is heated in a furnace where a sensor is kept above the metal ...
Text Solution
|
- Which statement is incorrect ?
Text Solution
|
- Heat given to a body which raises its temperature by 1^@C is
Text Solution
|
- Infrared radiation is detected by
Text Solution
|
- Which of the following is more close to a black body?
Text Solution
|
- Cooking gas container are kept in a lorry moving with uniform speed. T...
Text Solution
|
- If mass-energy equilivalence is taken into account, when water is cool...
Text Solution
|
- At what temperature is the r.m.s velocity of a hydrogen molecule equal...
Text Solution
|
- Even Carnot engine cannot give 100% efficiency because we cannot
Text Solution
|
- 1 mole of a gas with gamma=7//5 is mixed with 1 mole of a gas with gam...
Text Solution
|
- Two spheres of the same material have radii 1m and 4m and temperatures...
Text Solution
|
- "Heat cannot by itself flow from a body at lower temperature to a body...
Text Solution
|
- During an adiabatic process, the pressure of a gas is found to be prop...
Text Solution
|
- Which of the following parameters does not characterize the thermodyna...
Text Solution
|
- A Carnot engine takes 3xx10^6cal. of heat from a reservoir at 62^@C, a...
Text Solution
|
- The earth radiates in the infra-red region of the spectrum. The spectr...
Text Solution
|