A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUNIL BATRA (41 YEARS IITJEE PHYSICS)-HEAT AND THERMODYNAMICS-JEE Main And Advanced
- Which of the following is incorrect regarding the first law of thermod...
Text Solution
|
- The figure shows a system of two concentric spheres of radii r1 and r2...
Text Solution
|
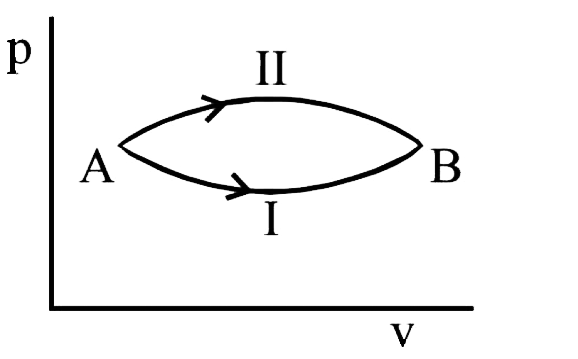
- A system goes from A and B via two processes. I and II as shown in fig...
Text Solution
|
- The temperature -entropy diagram of a reversible engine cycle is given...
Text Solution
|
- A gaseous mixture consists of 16g of helium and 16 g of oxygen. The ra...
Text Solution
|
- Assuming the Sun to be a spherical body of radius R at a temperature o...
Text Solution
|
- Two rigid boxes containing different ideal gases are placed on a table...
Text Solution
|
- The work of 142kJ is performed in order to compress one kilo mole of g...
Text Solution
|
- When a system is taken from state i to state f along the path iaf, it ...
Text Solution
|
- A Carnot engine, having an efficiency of eta=1//10 as heat engine, is ...
Text Solution
|
- One end of a thermally insulated rod is kept at a temperature T1 and t...
Text Solution
|
- If C(P and C(v) denote the specific heats nitrogen per unite mass at c...
Text Solution
|
- The speed of sound in oxygen (O2) at a certain temperature is 460ms^-1...
Text Solution
|
- An insulated container of gas has two chambers separated by an insulat...
Text Solution
|
- A long metallic bar is carrying heat from on eof its ends to the other...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- One kg of a diatomic gas is at pressure of 8xx10^4N//m^2. The density ...
Text Solution
|
- Statement-1: The temperature dependence of resistance is usually given...
Text Solution
|