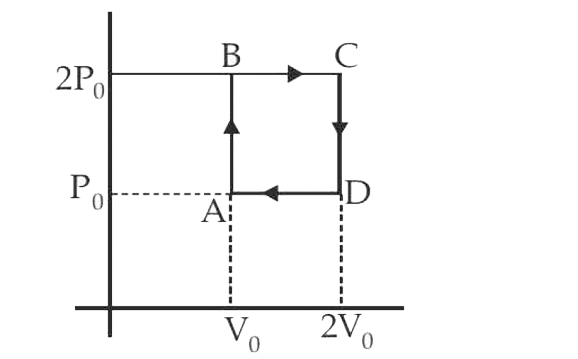
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUNIL BATRA (41 YEARS IITJEE PHYSICS)-HEAT AND THERMODYNAMICS-JEE Main And Advanced
- A themallly insulated vessel contains an ideal gas of molecular mass M...
Text Solution
|
- Three perfect gases at absolute temperature T1,T2, and T3 are mixed. T...
Text Solution
|
- A Carnot engine operating between temperature T1 and T2 has efficiency...
Text Solution
|
- 100g of water is heated from 30^@C to 50^@C. Ignoring the slight expan...
Text Solution
|
- A wooden wheel of radius R is made of two semicircular part . The two ...
Text Solution
|
- Helium gas goes through a cycle ABCDA (consisting of two isochoric and...
Text Solution
|
- A liquid in a beaker has temperature theta(t) at time t and theta0 is ...
Text Solution
|
- A Carnot engine, whose efficiency is 40%, takes in heat from a source ...
Text Solution
|
- The above p-v diagram represents the thermodymic cycle of an engine, o...
Text Solution
|
- If a piece of metal is heated to temperature theta and the allowed to ...
Text Solution
|
- The pressure that has to be applied to the ends of a steel wire of len...
Text Solution
|
- There is a circular tube in a vertical plane. Two liquids which do not...
Text Solution
|
- Three rods of Copper, Brass and Steel are welded together to from a Y ...
Text Solution
|
- One mole of a diatomic ideal gas undergoes a cyclic process ABC as sho...
Text Solution
|
- A solid body of constant heat capacity 1J//^@C is being heated by keep...
Text Solution
|
- Consider a spherical shell of radius R at temperature T. The black bod...
Text Solution
|
- Consider an ideal gas confined in an isolated closed chamber. As the g...
Text Solution
|
- n' moles of an ideal gas undergoes a process AtoB as shown in the figu...
Text Solution
|
- A pendulam clock loses 12s a day if the temperature is 40^@C and gains...
Text Solution
|
- An ideal gas under goes a quasi static, reversible process in whichh i...
Text Solution
|