Text Solution
Verified by Experts
Topper's Solved these Questions
SOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY|Exercise Ex 1.1 Objective Questions|6 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY|Exercise Ex 1.1 Objective Questions (Single Correct)|3 VideosS-BLOCK GROUP 2 - ALKALINE EARTH METALS
CENGAGE CHEMISTRY|Exercise Ex 5.1 Objective|2 VideosSTATES OF MATTER
CENGAGE CHEMISTRY|Exercise Exercises (Ture False)|25 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-SOME BASIC CONCEPTS AND MOLE CONCEPT-Archives Subjective
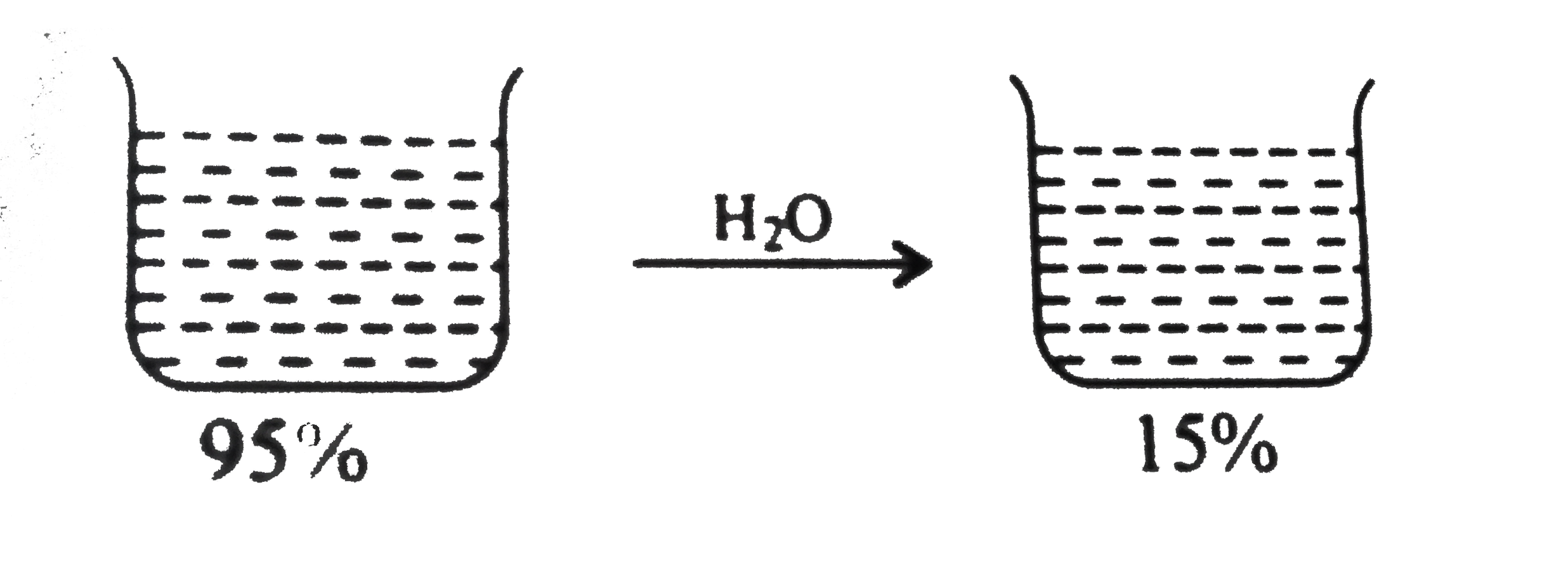
- What volume of 95% H(2) SO(4) by weight (d = 1.85 g mL^(-1)) and what ...
Text Solution
|
- What is the molarityk and molality of a 13% solution (by weight) of su...
Text Solution
|
- Calculate the density of NH(3) at 30^(@)C and 5 atm pressure.
Text Solution
|
- When 4.215 g of a metallic carbonate was heated in a hard glass tube, ...
Text Solution
|
- The density of 3 M sodium of thiosulphate solution (Na(2)S(2)O(3)) is ...
Text Solution
|
- A sugar syrup of weight 214.2 g contains 34.2 g of sugar (C(12) H(22) ...
Text Solution
|
- Calculate the molality of 1 L solution of 93% H(2)SO(4) (Weight/volume...
Text Solution
|
- Calculate the volume occupied by 5.0 g of acetylene gas at 50^(@)C and...
Text Solution
|
- On mixing 45.0 mL of 0.25 M lead nitrate solution with 25.0 mL of 0.10...
Text Solution
|
- When 0.575 xx 10^(-2) kg of Glaube's salt is dissolved in water, we ge...
Text Solution
|
- Calculate the molarity of water if its density is 1000 kg m^(-3)
Text Solution
|
- Calculate the amount of calcuium oxide required when it reacts with 85...
Text Solution
|