Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY|Exercise Exercises Subjective (Limiting Reagent)|2 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY|Exercise Exercises Subjective (Empirical And Molecular Formulae)|4 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY|Exercise Ex 1.2 Fill In The Blanks|1 VideosS-BLOCK GROUP 2 - ALKALINE EARTH METALS
CENGAGE CHEMISTRY|Exercise Ex 5.1 Objective|2 VideosSTATES OF MATTER
CENGAGE CHEMISTRY|Exercise Exercises (Ture False)|25 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-SOME BASIC CONCEPTS AND MOLE CONCEPT-Exercises Subjective (Laws Of Chemical Combination)
- 10 mL of hydrogen combine with 5mL of oxygen to yield water. When 200 ...
Text Solution
|
- Common salt obtained from Clifton beach contained 6.75% chlorime while...
Text Solution
|
- 3.2 g sulphur combines with 3.2 g of oxygen, to from a compound in one...
Text Solution
|
- 1 g of oxygen combines with 0.1260 g of hydrogen to form H(2)O. 1 g o...
Text Solution
|
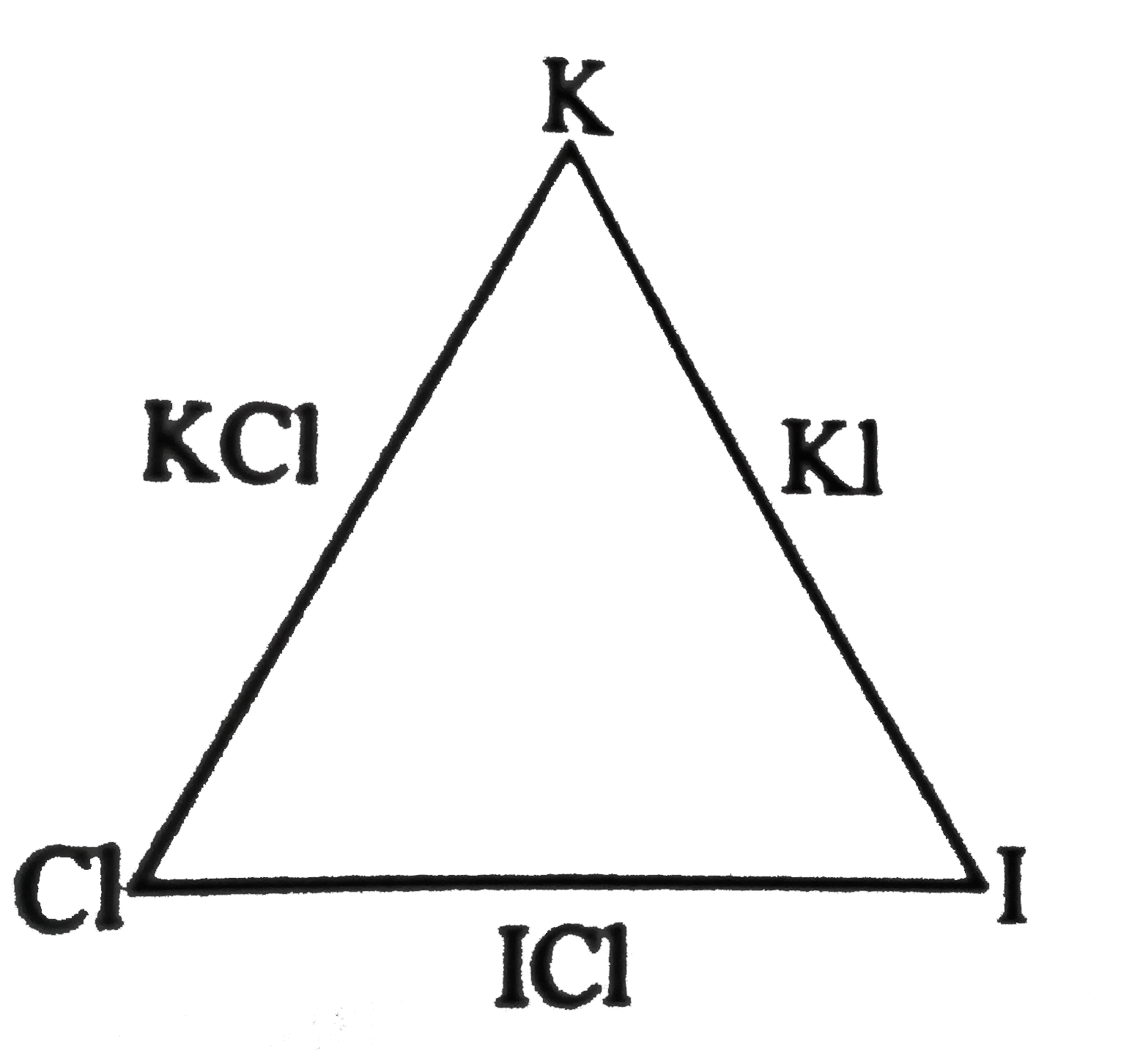
- KCL contains 52 % of potassium, Kl contains 23.6% of potassium, and IC...
Text Solution
|
- What weight of sodium chloride would be decomposed by 4.9 g of sulphri...
Text Solution
|
- If the law of constant compositon is true, what weights of calcium car...
Text Solution
|
- An element forms two oxides containing, 50% & 40% of oxygen respective...
Text Solution
|
- Elements A and B combine to form three different compounds: 0.3 g of...
Text Solution
|
- An impure sample of sodium chloride that weighed 0.50 g gave 0.90 g of...
Text Solution
|
- How much magnesium sulphide can be obtained from 2.00 g of Mg and 2.00...
Text Solution
|
- 1.00 g of a hydrated salt contains 0.2014 g of iron, 0.1153 g fo sulph...
Text Solution
|
- A compound on analysis gave the following percentage composition by we...
Text Solution
|