Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY-FLUID MECHANICS-Exercise16.1
- Water and oil are poured into the two limbs of a U-tube containing mer...
Text Solution
|
- The liquids shown in figure in the two arms are mercury (specific grav...
Text Solution
|
- The heights of mercury surfaces in the tow arms of the manometer shown...
Text Solution
|
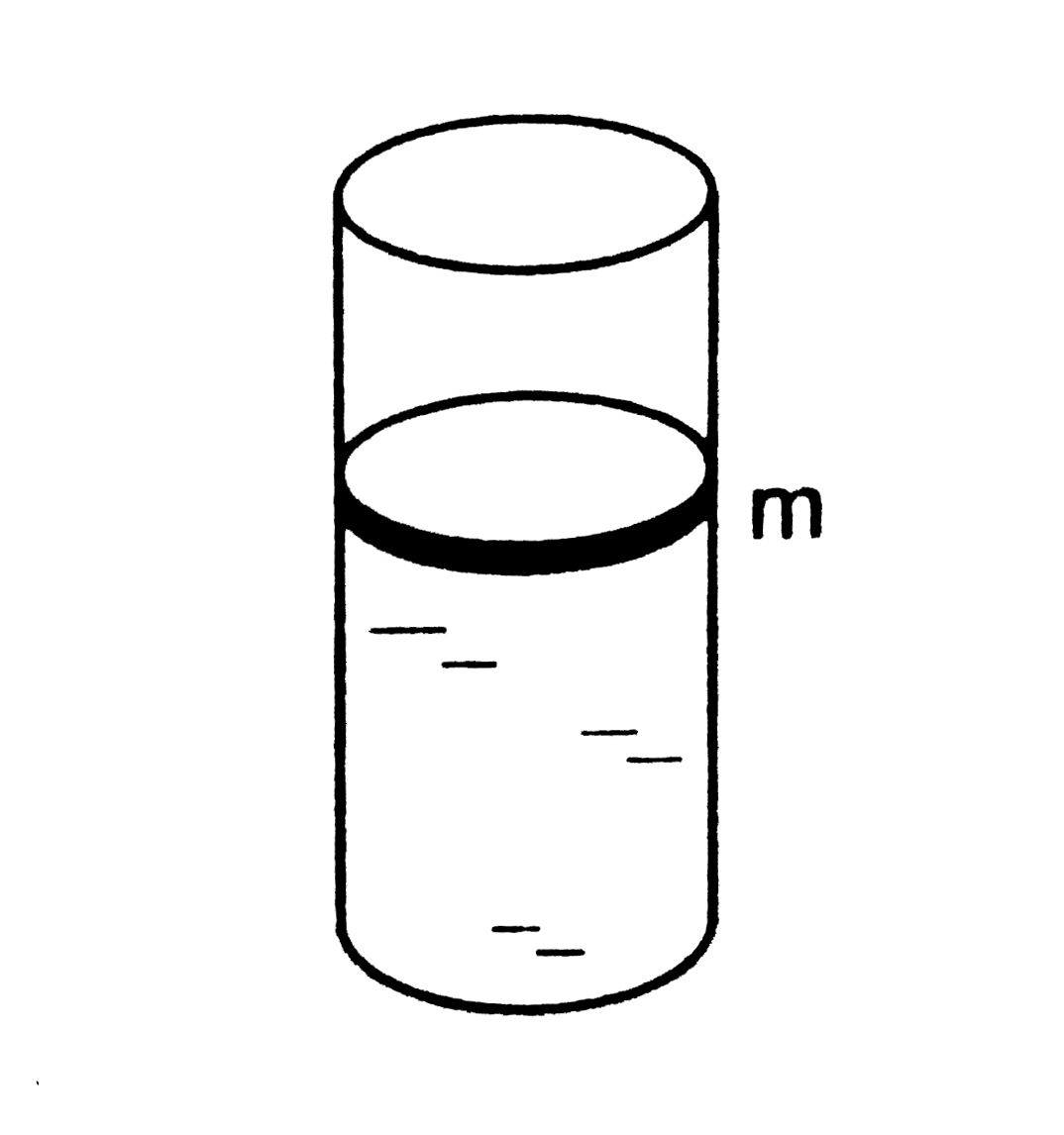
- A cylidrical vesel containing a liquid is closed by a smooth piston o...
Text Solution
|
- The area of cross section of the two arms of a hydraulilc press are 1 ...
Text Solution
|
- The area of cross section of the wider tube shown in figure is 900 cm^...
Text Solution
|
 ,
,