Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASES
DC PANDEY|Exercise Exercise 20.4|3 VideosTHERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASES
DC PANDEY|Exercise Exercise 20.5|7 VideosTHERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASES
DC PANDEY|Exercise Exercise 20.2|7 VideosTHERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES
DC PANDEY|Exercise Medical entrance gallary|30 VideosUNIT AND DIMENSIONS
DC PANDEY|Exercise Assertion And Reason|2 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY-THERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASES-Exercise 20.3
- From the graph for an ideal gas, state whether m1 or m2 is greater. ...
Text Solution
|
- A vessel is filled with an ideal gas at a pressure of 20 atm and is a ...
Text Solution
|
- A vessel contains a mixture of 7 g of nitrogen and 11 g carbon dioxide...
Text Solution
|
- An electric bulb of volume 250cc was sealed during manufacturing at a ...
Text Solution
|
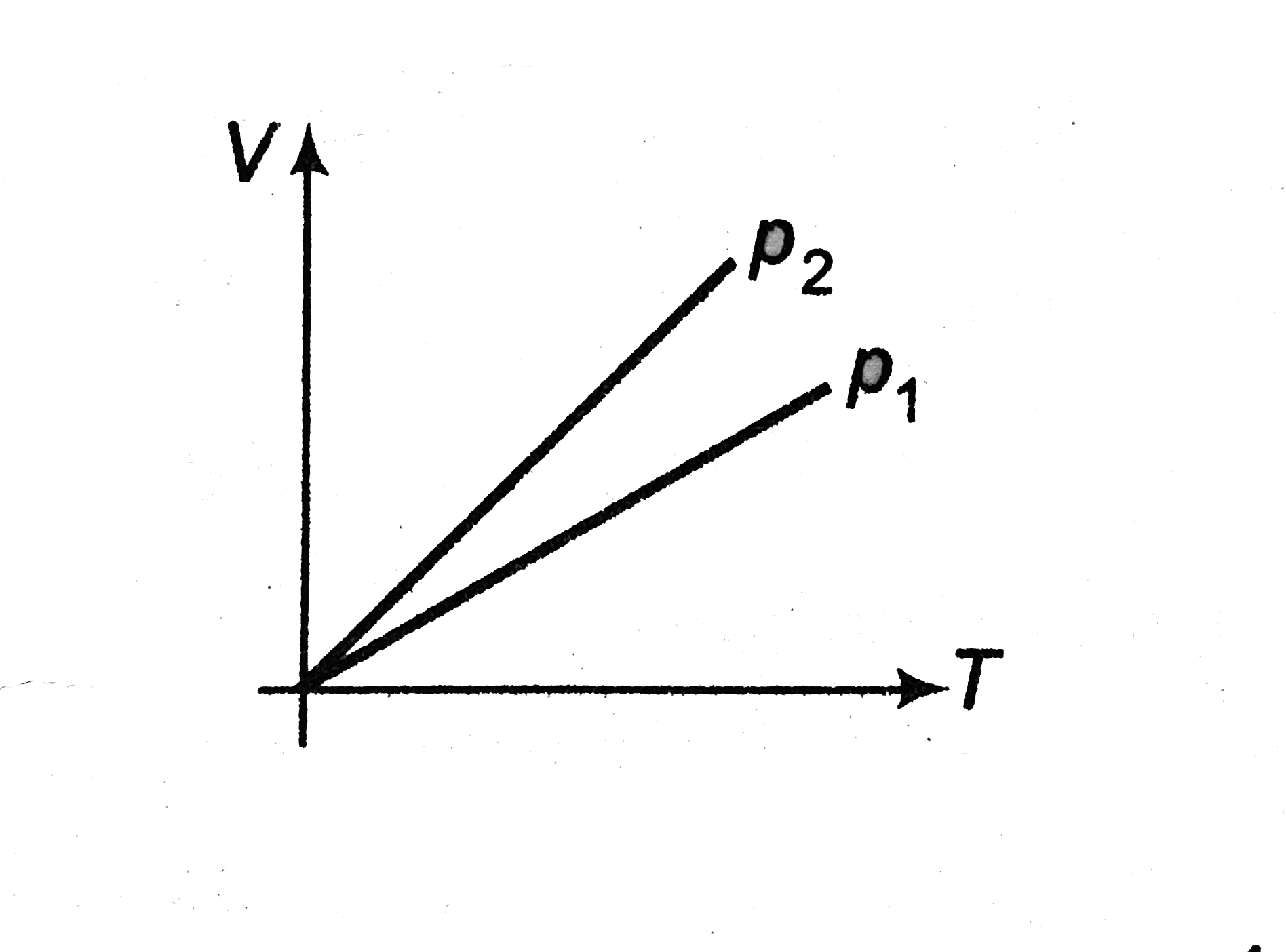
- State whether (p1 gt p2 or p2 gt p1) for given mass of a gas ? .
Text Solution
|
- For a given mass of a gas what is the shape of (p) versus (1/V) graph ...
Text Solution
|
- For a given mass of a gas, what is the shape of (pV) versus (T) graph ...
Text Solution
|
 .
.