A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AIIMS PREVIOUS YEAR PAPERS-AIIMS 2005-CHEMISTRY
- Assertion: Reaction of SO(2) and H(2)S in the presence of Fe(2)O(3) ca...
Text Solution
|
- Assertion: SiF(6)^(2-) is known but SiCl(6)^(2-) is not. Reason: Siz...
Text Solution
|
- Borax bead test is not suitable of for Al(III) Al(2)O(3) is insolubl...
Text Solution
|
- Statement -1 : Ozone is a powerful oxidising agent in comparison to O(...
Text Solution
|
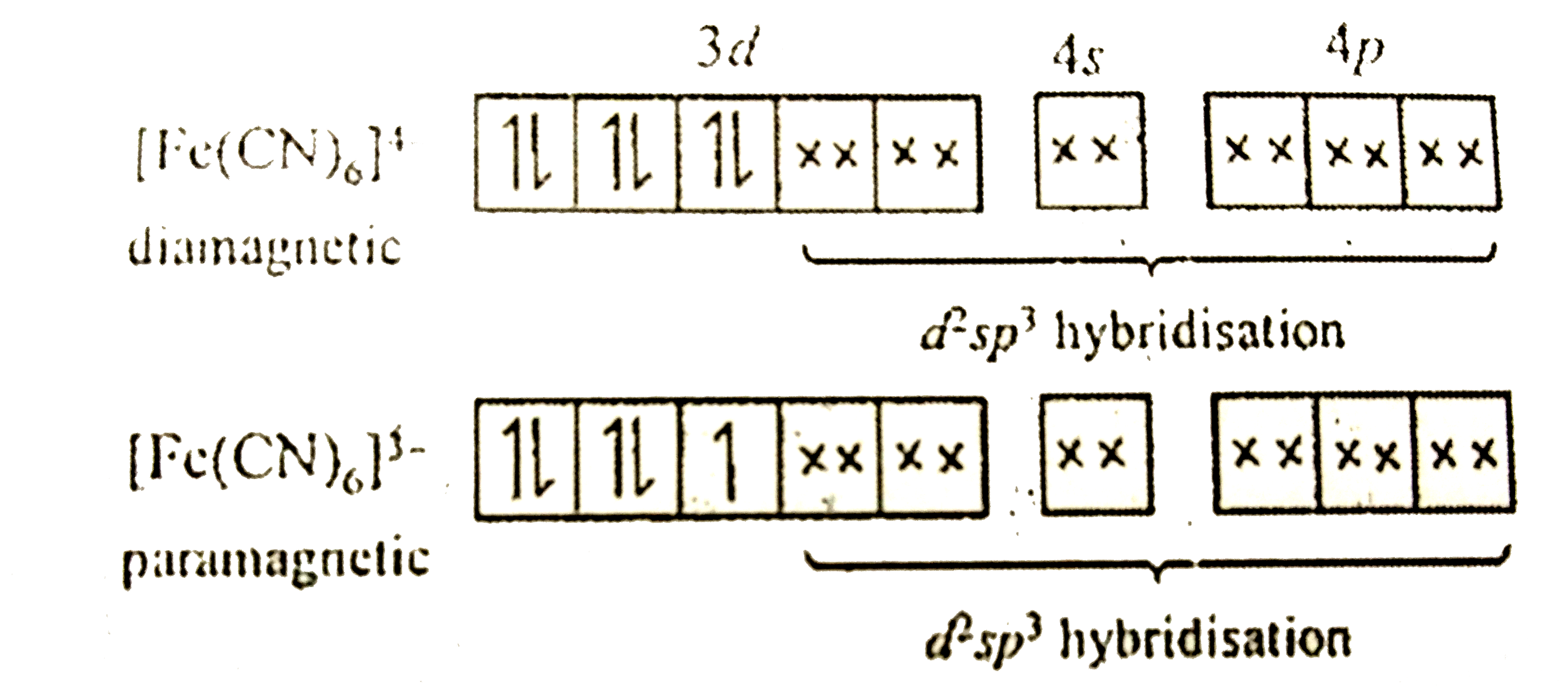
- Assertion : Potassium ferrocyanide is diamagnetic whereas potassium fe...
Text Solution
|
- Assertion (A): Addtion of NH(4)OH to an aqueous solution of BaCl(2) in...
Text Solution
|
- Asseration: SeCl(4), does not havea tetrahedral structure. Reason: S...
Text Solution
|
- Each question contains STATEMENT-I(Assertion) and STATEMENT-2(Reason)....
Text Solution
|
- Assertion: Compressibility factor for hydrogen varies with pressure wi...
Text Solution
|
- Assertion :First ionization energy is lower than oxygen. Reason :Ac...
Text Solution
|
- Assertion B(2) molecule is diamagnetic Reasoning The highest occupie...
Text Solution
|
- Assertion : Rate of hydrolysis of methyl chloride to methanol is highe...
Text Solution
|
- Assertion (A) : Galvanized iron does not rust. Reason (R): Zn has a...
Text Solution
|
- Statement-I : Extraction of iron metal from iron oxide ore is carried ...
Text Solution
|
- (A) Rate of nitration of benzene and hexadeuterobenzene are different....
Text Solution
|
- Assertion. t-Butyl Methyl ether is not prepared by the reaction of t-b...
Text Solution
|
- Assertion:Maltose is a reducing sugar , one molecule of which gives tw...
Text Solution
|
- (A) p-O(2)N-C(6)H(4)COCH(3) is prepared by Friedel Crafts acylation of...
Text Solution
|
- Assertion : Alkyl isocyanides in acidified water give alkyl formamides...
Text Solution
|
- Assertion : Cyclopentadienyl anion is much more stable than allyl anio...
Text Solution
|