Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Ex 2.2|18 VideosREDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Exercise|29 VideosREDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Archives (Integers)|1 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY|Exercise Assertion Reasoning Type|5 VideosS-BLOCK GROUP 1 - ALKALI METALS
CENGAGE CHEMISTRY|Exercise Archives Subjective|8 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-REDOX REACTIONS-Ex 2.1
- Identify the oxidant and the reductant in the following reactions: a...
Text Solution
|
- Find the oxidation number of sulphur in the following compounds: H(2)S...
Text Solution
|
- Find the oxidation number of Cl in HCl, HClO,ClO(4)^(ө), and Ca(Ocl)Cl...
Text Solution
|
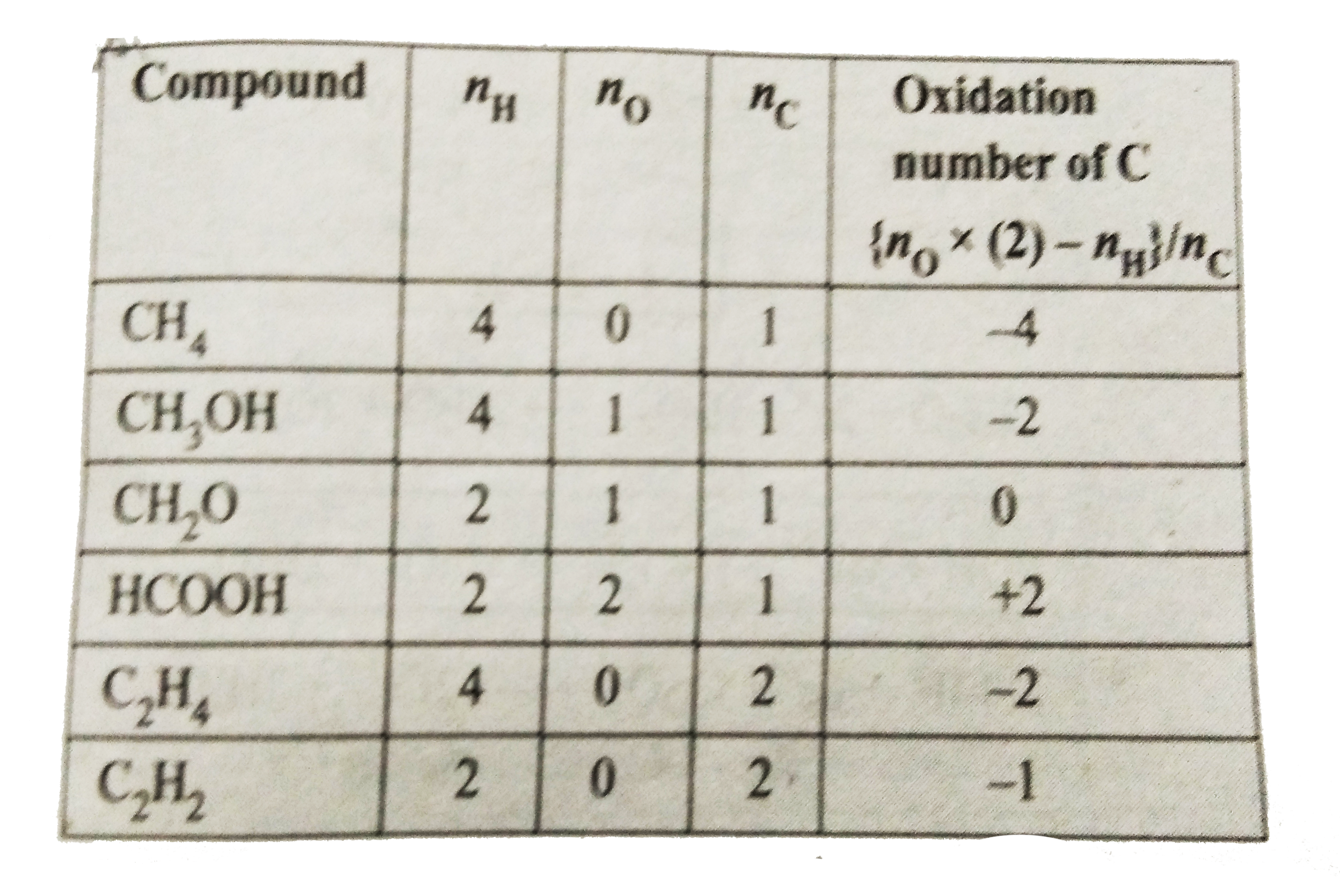
- Find the oxidation number of carbon in the following compounds: CH(3)O...
Text Solution
|
- Find the oxidation number of Fe in Fe(3)O(4) and in Fe(III)(4)[Fe(II)(...
Text Solution
|
- Identify the oxidant and reductant in the following reactions: a. 10...
Text Solution
|
- Identify the species undergoing oxidation and reduction. a. H(2)S(g)...
Text Solution
|
- Justify that the reaction 2Cu(2)O(s)+Cu(2)S(s)rarr6Cu(s)+SO(2)(g) a ...
Text Solution
|
- Which of the following represents oxidation? a. NO(2)^(ө)rarrN(2), b...
Text Solution
|
- Using stock notation, represent the following compounds and write thei...
Text Solution
|
 .
.