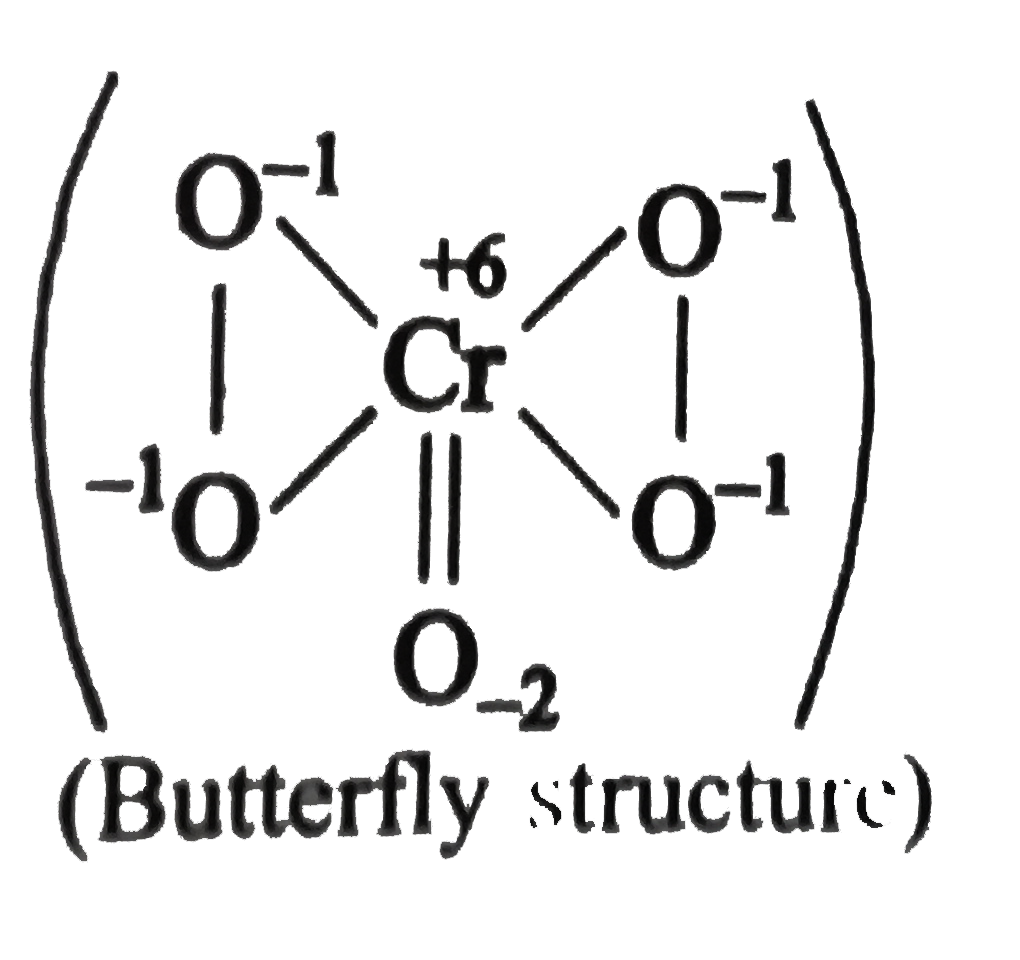
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STOICHIOMETRY
CENGAGE CHEMISTRY|Exercise Exercises Single Correct|85 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY|Exercise Exercises Assertion Reasoning|15 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY|Exercise Exercises Linked Comprehension|42 VideosSTATES OF MATTER
CENGAGE CHEMISTRY|Exercise Exercises (Ture False)|25 VideosTHERMODYNAMICS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|23 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-STOICHIOMETRY-Exercises Multiple Correct
- Which of the following statements about the following reaction is//are...
Text Solution
|
- 100 " mL of " (M)/(10) Ca(MnO(4))(2) in acidic medium can be oxidised ...
Text Solution
|
- Which of the following statements is/are correct about the reaction. ...
Text Solution
|
- 0.1 " mol of "MnO(4)^(ɵ) (in acidic medium) can:
Text Solution
|
- H(2)C(2)O(4) and NaHC(2)O(4) behave as acids as well as reducing agent...
Text Solution
|
- A compound contains three elements A,B and C, if the oxidation number ...
Text Solution
|
- Choose the correct statement:
Text Solution
|
- For the following balanced redox reaction: 2MnO(4)^(ɵ)+8H^(o+)+Br(2)...
Text Solution
|
- Which of the following statements is/are correct about the followig re...
Text Solution
|
- Which of the following statements is//are correct about 6.8% stregnth ...
Text Solution
|
- Which of the following reactions is/are not intermolecular redox react...
Text Solution
|
- 1 L sample of impure water containing sulphide ion is made ammoniacal ...
Text Solution
|
- Which of the following statements is/are correct about the reaction. ...
Text Solution
|
- Which of the following statements is/are correct in the following reac...
Text Solution
|
- In which of the reaction,oxygen is an oxidant.
Text Solution
|
- 56.0 g KOH, 138.0 g K(2)CO(3) and 100.0 g KHCO(3) is dissolved in wate...
Text Solution
|
- x g of H2O2 requires 100mL of M//5 KMnO4 in a titration in a solution ...
Text Solution
|
- A mixture of n(1) moles of Na(2)C(2)O(4) and NaHC(2)O(4) is titrated s...
Text Solution
|
- 100 " mL of " 0.2 M Kal(OH)(2)CO(3) solution is completely neutralised...
Text Solution
|
- Which of the following is/are correct about the redox reaction? MnO(...
Text Solution
|