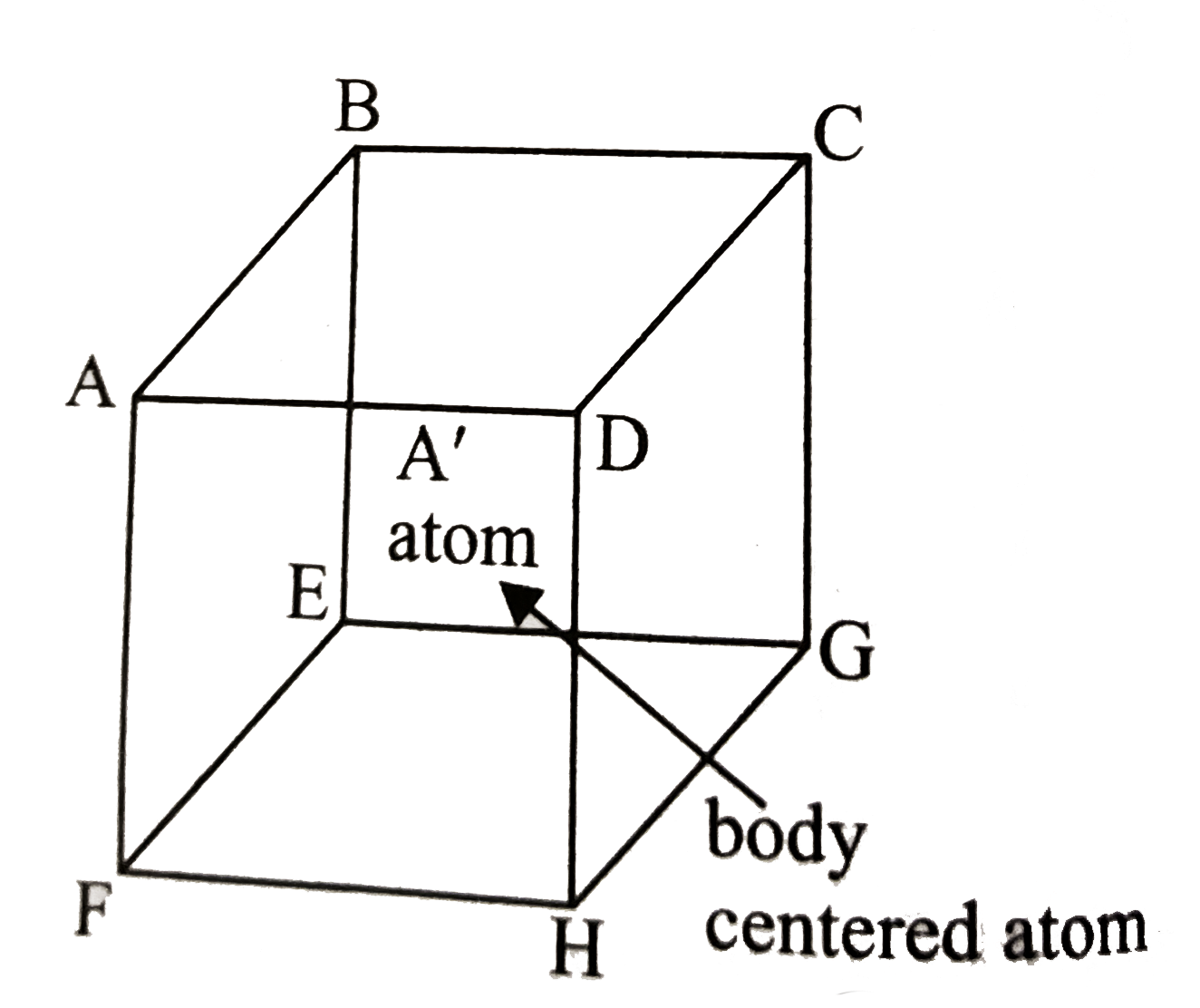
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
CENGAGE CHEMISTRY|Exercise Exercises (Assertion-Reasoning)|19 VideosSOLID STATE
CENGAGE CHEMISTRY|Exercise Exercises (Interger)|9 VideosSOLID STATE
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correct)|39 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise SUBJECTIVE TYPE|3 VideosSOLUTIONS
CENGAGE CHEMISTRY|Exercise Ex 2.3 (Objective)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-SOLID STATE-Exercises (Single Correct)
- A metal crystallizes in bcc lattice. The percent fraction of edge leng...
Text Solution
|
- In the cubic lattice given below, the three distances between the atom...
Text Solution
|
- In body-centred cubic lattice given below, the three disntances AB, AC...
Text Solution
|
- Two ionic solids AB and CB crystallize in the same lattice. If r(A^(o+...
Text Solution
|
- A molecule A(2)B (Mw = 166.4) occupies triclinic lattice with a = 5 Å,...
Text Solution
|
- Silicon dopped with group 13 and group 15 member element is, repective...
Text Solution
|
- Na and Mg crystallize in bcc- and fcc-type crystals, respectively, the...
Text Solution
|
- The electrical conductivity of semiconductor is
Text Solution
|
- Pure silicon and germanimum are
Text Solution
|
- A solid has a structure in which W atoms are located at the corners of...
Text Solution
|
- Which of the following is a ferroelectric compound?
Text Solution
|
- The intermetallic compound LiAg crystallizes in cubic lattice in which...
Text Solution
|
- The edge length of a face-centred cubic unit cell is 508 pm. If the ra...
Text Solution
|
- In the crystals of which of the following ionic compounds would you ex...
Text Solution
|
- Schottky defect to crystals is observed when
Text Solution
|
- How many kinds of space lattices are possible in a crystal?
Text Solution
|
- Potassium crystallizes with a
Text Solution
|
- A compound formed by element A and B crystallizes in the cubic structu...
Text Solution
|
- The number of unit cells in 58.5 g of NaCl is nearly
Text Solution
|
- The number of octahedral sites per sphere in fcc structure is
Text Solution
|