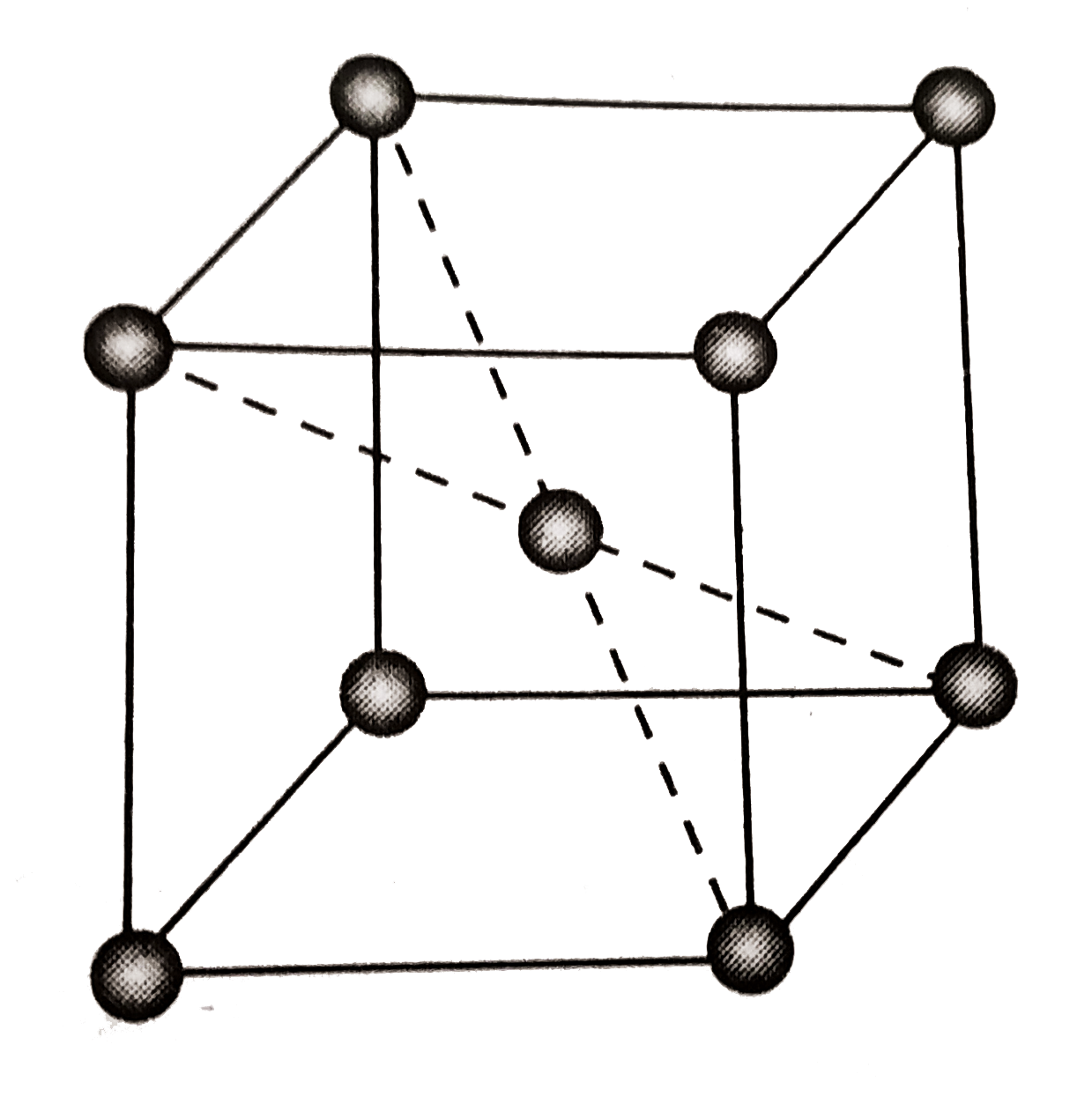
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
CENGAGE CHEMISTRY|Exercise Exercises (Archives ) Single Correct|10 VideosSOLID STATE
CENGAGE CHEMISTRY|Exercise Exercises (Archives ) Assertion-Reasoning|1 VideosSOLID STATE
CENGAGE CHEMISTRY|Exercise Exercises (Archives ) Linked Comprehension|3 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise SUBJECTIVE TYPE|3 VideosSOLUTIONS
CENGAGE CHEMISTRY|Exercise Ex 2.3 (Objective)|9 Videos
Similar Questions
Explore conceptually related problems