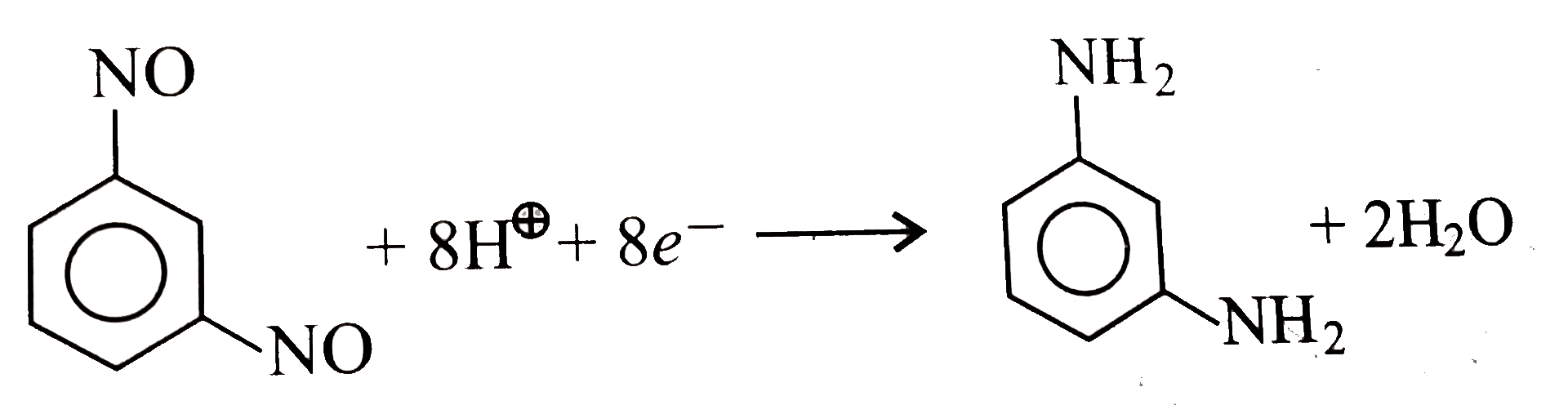Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Ex 3.1 (Objective)|28 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Ex 3.2 (Objective)|26 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Solved Examples (Electrochemical Cell)|36 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ELECTROCHEMISTRY-Solved Examples(Electrolysis And Electrolytic Cells)
- Express each of the following combinations of electrical units as a si...
Text Solution
|
- A resistance heater was wound around a 5.0g metallic cylinder. A curre...
Text Solution
|
- In a zinc manganese dioxide dry cell, the anode is madeup of Zn1 and ...
Text Solution
|
- An aqueous solution of NaCl on electrolysis gives H(2)(g), Cl(2)(g), a...
Text Solution
|
- A constant current was flowen for 1 mi n through a solution of Kl . At...
Text Solution
|
- Aqueous solution of m- dinitroso benzene was electrolyzed for 2 hors p...
Text Solution
|
- A 35% solution of LiCl was electrolyzed by using a 2.5 A current for 0...
Text Solution
|
- A current of 10A is employed to plate nickel in NiSO(4) bath. The curr...
Text Solution
|
- A certain amount of charge is passed through acidulated water. A total...
Text Solution
|
- During the electrolysis of water, a total volume of 33.6mL of hydrogen...
Text Solution
|
- During an electrolysis of conc H(2)SO(4) , perdisulphuric acid (H(2)S(...
Text Solution
|
- A current of 1.0A is passed for 96.5s trhough a 200mL solution of 0.05...
Text Solution
|