Text Solution
Verified by Experts
Topper's Solved these Questions
SURFACE CHEMISTRY
CENGAGE CHEMISTRY|Exercise Ex 5.1|11 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY|Exercise Ex 5.2|11 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY|Exercise Archives Subjective|2 VideosSOLUTIONS
CENGAGE CHEMISTRY|Exercise Ex 2.3 (Objective)|9 VideosSYNTHETIC AND NATURAL POLYMERS
CENGAGE CHEMISTRY|Exercise Exercises Assertion Reasoning|10 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-SURFACE CHEMISTRY-Solved Examples
- Write two differences between multimolecular colloids and macromolecul...
Text Solution
|
- Physical and chemical adsorption respond differenlty with a rise in te...
Text Solution
|
- A small amount of silica gel and anhydrous calcium chloride are placed...
Text Solution
|
- How is adsorption of a gas related to its critical temperature?
Text Solution
|
- Explain the following observation: a. Lyphilic colloid is more stabl...
Text Solution
|
- Give one test to distinguish whether the given emulsion is oil-in-wate...
Text Solution
|
- Consider the adsorption isotherm given below and interpret the variati...
Text Solution
|
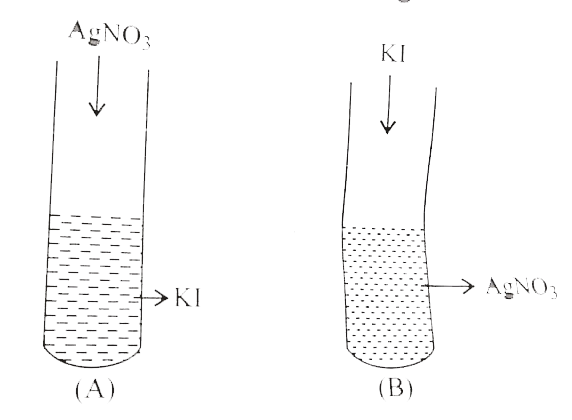
- A colloidal solution of Agl is prepared by two different methods as sh...
Text Solution
|
- Adsorption Theory Of Heterogeneous Catalysis
Text Solution
|
- Adsorption, if spontaneous, is exothermic. Explain.
Text Solution
|
- In a coagulation experiment, 5mL of As(2)S(3) is mixed with distilled...
Text Solution
|
- In an adsorption experiment, a graph between log (x/m) versus log P wa...
Text Solution
|
- The volume of nitrogen gas Vm (at STP) required to cover a sample of s...
Text Solution
|
- 100mL of a colloidal solution is completely precipitated by addition o...
Text Solution
|
- What is the charge on the colloidal particles in the following ? a. ...
Text Solution
|
- which of the following is most effective in coagulating ferric hydroxi...
Text Solution
|
- Write a mathematical expression showing relationship between the amoun...
Text Solution
|
- In the case of chemisorption, why adsorption first increases and then ...
Text Solution
|
- Why physisorption is multi-molecular whereas chemisorption is unimolec...
Text Solution
|
- What happens when a freshly precipitated Fe(OH)(3) is shaken with litt...
Text Solution
|