Text Solution
Verified by Experts
Topper's Solved these Questions
PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY|Exercise SOLVED EXAMPLES|17 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY|Exercise Subjective Type|11 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercises (Archives )Subjective|4 VideosREDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Archives (Integers)|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS-Assertion Reasoning Type
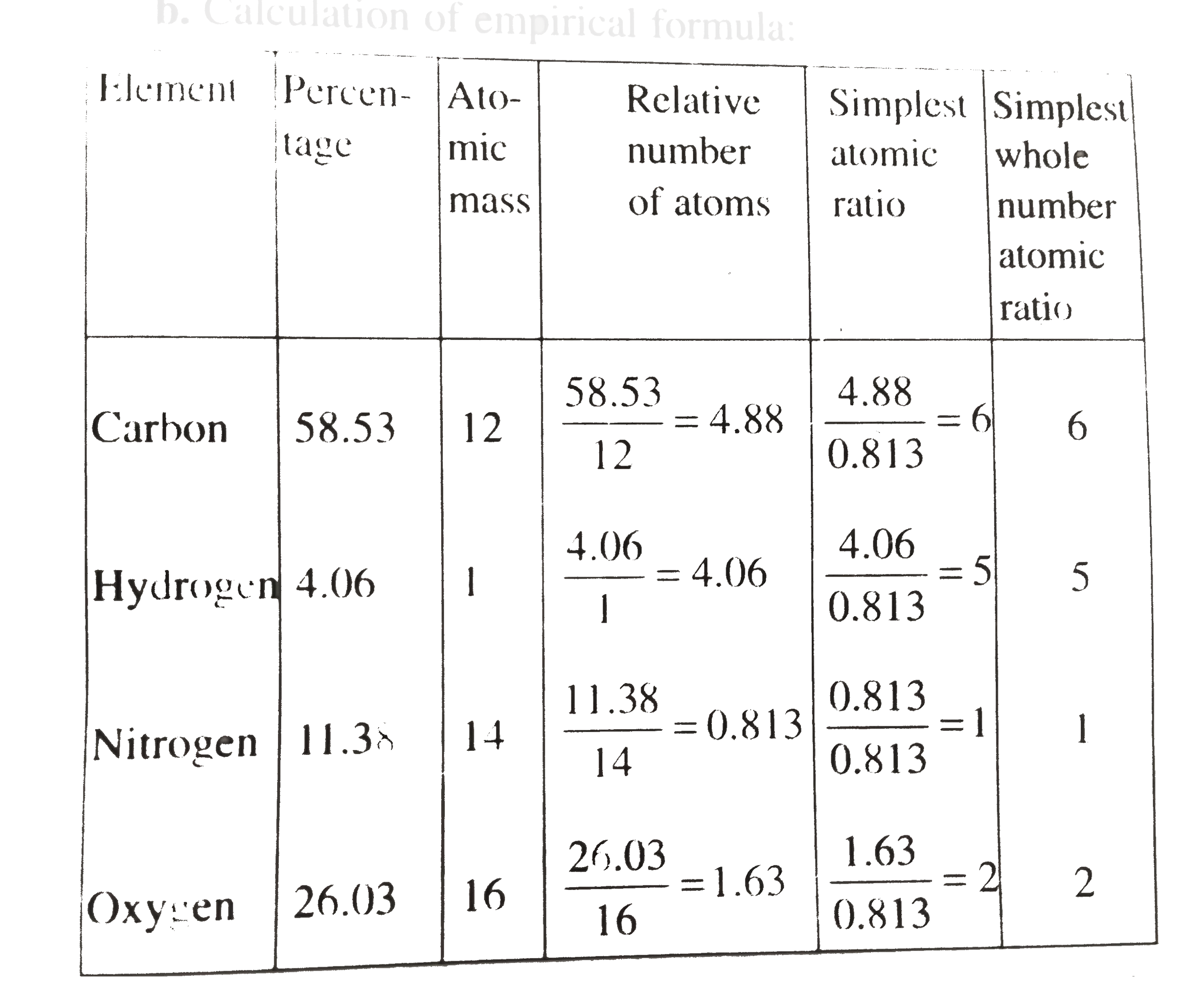
- 0.246 gm of an organic compound containing 58.53% carbon and 4.06% hyd...
Text Solution
|
- (a). If both (A) and (R) are correct and (R) is the correct explanatio...
Text Solution
|
- (a). If both (A) and (R) are correct and (R) is the correct explanatio...
Text Solution
|
- (a). If both (A) and (R) are correct and (R) is the correct explanatio...
Text Solution
|
- (a). If both (A) and (R) are correct and (R) is the correct explanatio...
Text Solution
|
- (a). If both (A) and (R) are correct and (R) is the correct explanatio...
Text Solution
|