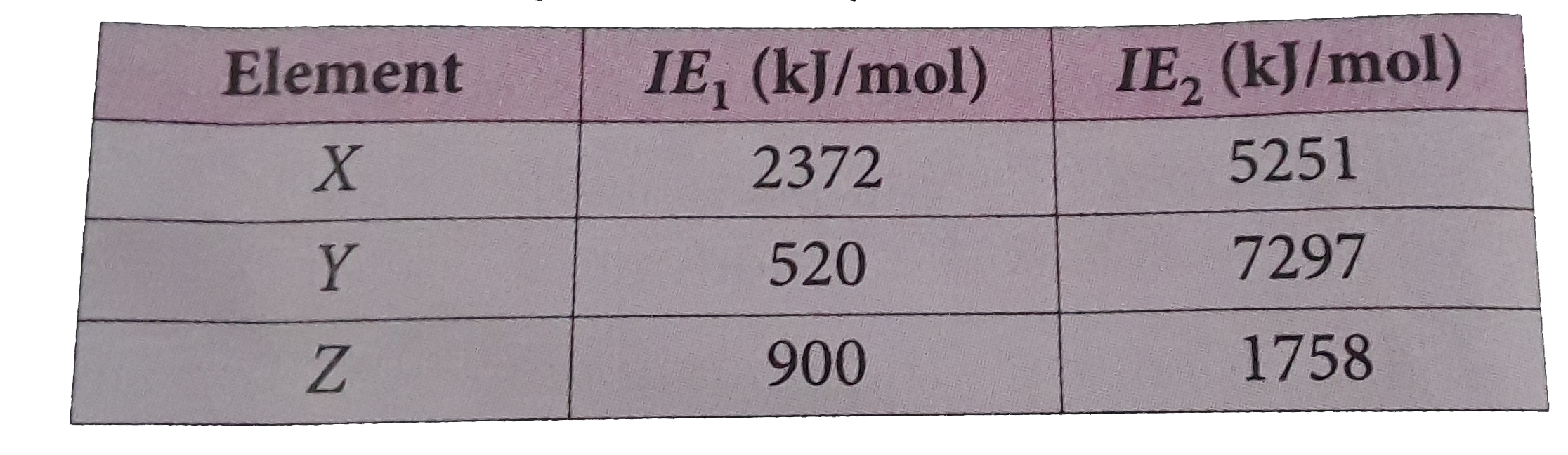
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY
NCERT FINGERTIPS|Exercise Higher Order Thinking Skills|10 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY
NCERT FINGERTIPS|Exercise NCERT Exemplar|12 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY
NCERT FINGERTIPS|Exercise Electronic Configuration And Type Of Elements|20 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosENVIRONMENTAL CHEMISTRY
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-CLASSIFICATION OF ELEMENTS AND PERIODICITY -Periodic Trends In Properties Of Elements
- Beryllium has higher ionisation enthalpy than boron.This can be explai...
Text Solution
|
- A sudden large jump between the values of second and third ionisation ...
Text Solution
|
- Few elements are matched with their successive ionisation energies,Ide...
Text Solution
|
- First and second ionisation enthalpies(in KJ/mol) of few elements are ...
Text Solution
|
- Ionization enthalpies of transition metals are
Text Solution
|
- Consider these electronic configurations for neutral atoms: (i) 1s^(...
Text Solution
|
- Which of the following element will have highest ionization energy?
Text Solution
|
- Few values of enthalpies are given below: O=-141 KJ mol^(-1) F= -328 K...
Text Solution
|
- In the given graph,a periodic property (R) is plotted against atomic n...
Text Solution
|
- Which is correct increasing order of their tendency of the given eleme...
Text Solution
|
- Which of the following have least electron affinity?
Text Solution
|
- Which one of the following arrangements represents the correct order o...
Text Solution
|
- Which of the following statements is not correct about the electron ga...
Text Solution
|
- Why is the electron gain enthalpy of O or F less than that of S or Cl?
Text Solution
|
- Which of the following statements regarding an anion is not true?
Text Solution
|
- Which of the following properties of isotopes of an element is differe...
Text Solution
|
- Given below are the names of new elements based on their position in t...
Text Solution
|
- Which of the following noble gases has the maximum positive electron g...
Text Solution
|
- An atom with high electronegativity has
Text Solution
|
- Study the given diagram of the periodic table and fill the blanks with...
Text Solution
|