Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise PROBLEMS|55 VideosELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT ANSWER QUESTIONS|63 VideosD AND F- BLOCK ELEMENTS AND CO-ORDINATION COMPOUNDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|33 VideosGENERAL PRINCIPLES OF MMETALLURGY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE SAQ|1 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ELECTROCHEMISTRY & CHEMICAL KINETICS-DAM SURE
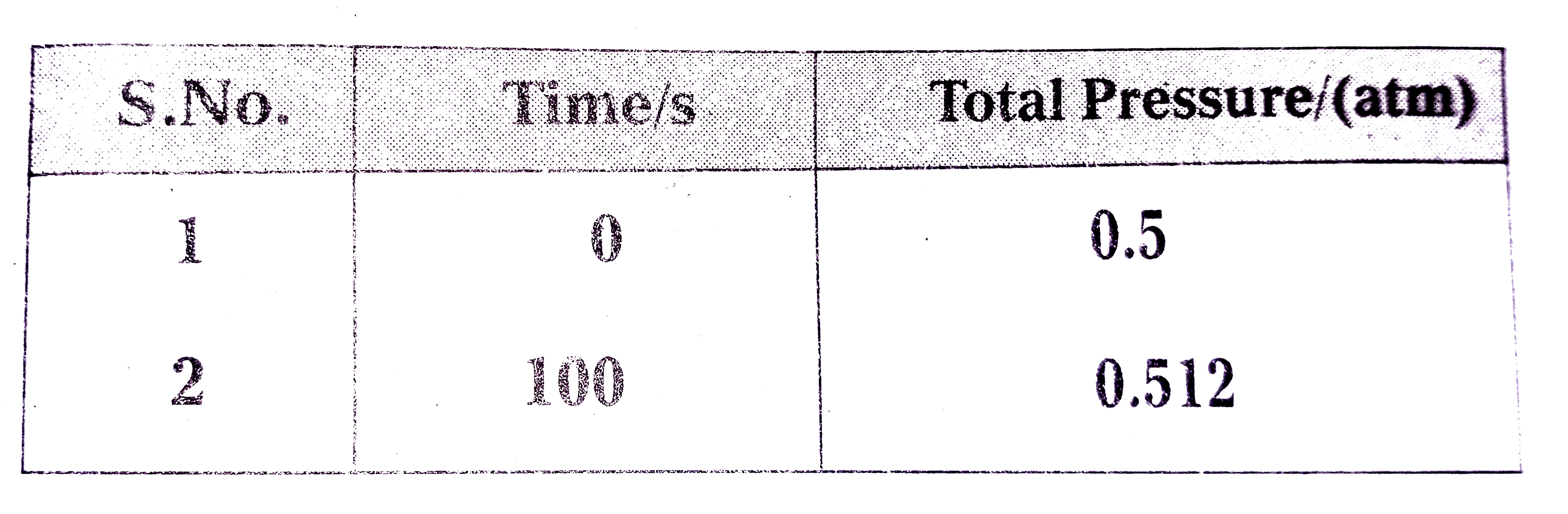
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- Diffine conductivity of a material. Give its SI units.
Text Solution
|
- State Faraday's seconed law of electroystis.
Text Solution
|
- State Kohlrausch's law of independent magration of ions.
Text Solution
|
- Define electrochemical equivalent (e.c.e).
Text Solution
|
- State and explain Kohlrausch's law of indendent migration of ions.
Text Solution
|
- Define emf. Calculat the emf of the following galvanic cell : Zn((s)...
Text Solution
|
- Write Nernst equation for a metal and non metal eletrode.
Text Solution
|
- What is Rate of a reaction
Text Solution
|
- What is Rate equation (or) Rate expression (or) Rate Law ?
Text Solution
|
- Write the difference between Order and Molecularity of a reaction.
Text Solution
|
- A first order reaction is found to have a rate constant, k=5.5 xx10^(-...
Text Solution
|
- What is Zero Order reaction ?
Text Solution
|
- What is First Order reaction ? Give example.
Text Solution
|
- What are pseudo first order reactions ? Give one example.
Text Solution
|
- What is Half life of a reaction ?
Text Solution
|
- Give two example for gaseous first order reactions.
Text Solution
|
- What is a second order reaction ? Give one example.
Text Solution
|
- Explain the factors influencing rate of reaction.
Text Solution
|