Text Solution
Verified by Experts
Topper's Solved these Questions
REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Exercise (Linked Comprehension)|52 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Exercise (Multiple Correct)|35 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise EXAMPLE|1 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY|Exercise Viva Voce Questions And Part-C (Analysis Of Cations)|42 VideosSOLID STATE
CENGAGE CHEMISTRY|Exercise Ex 1.2 (Objective)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS-Exercise
- Complete the follwing:
Text Solution
|
- Convert (Note that KMnO(4) can cause cleavage of the ring in the pr...
Text Solution
|
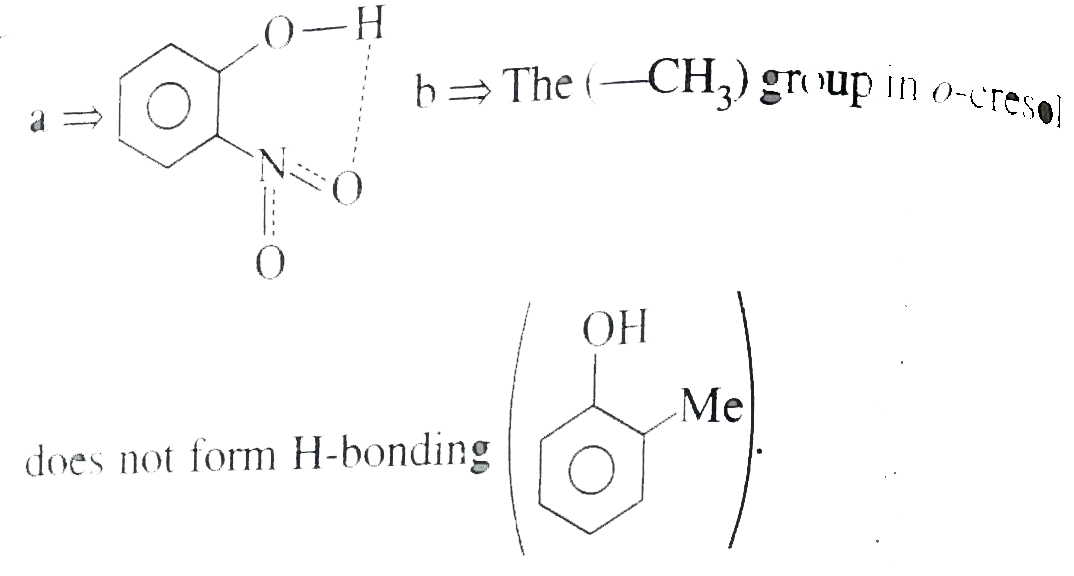
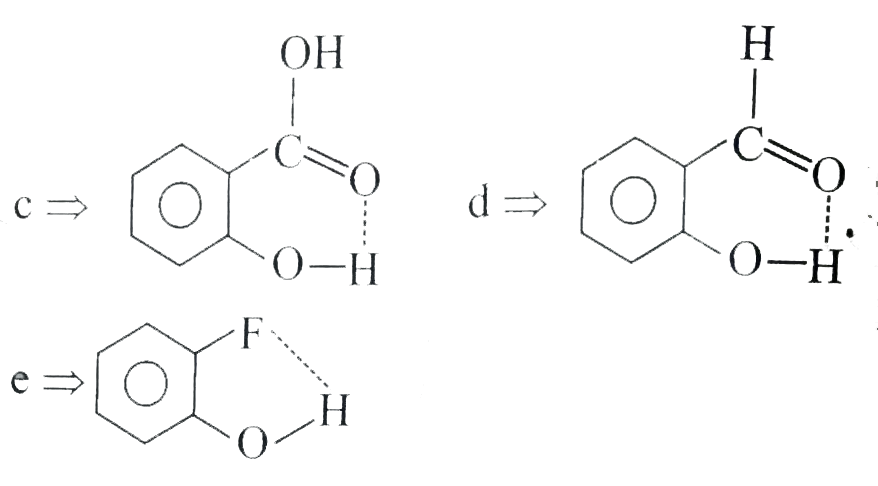
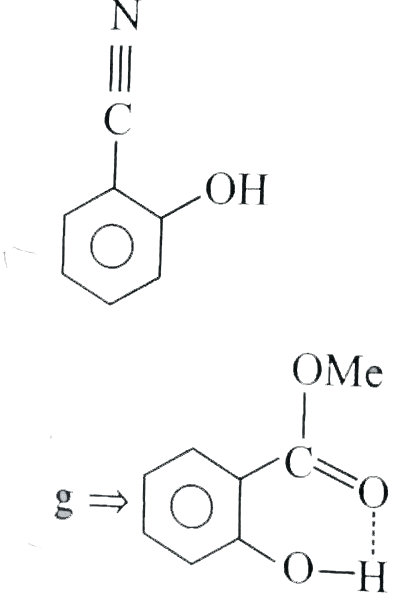
- In which of the following compounds does intramolecular H-bonding occu...
Text Solution
|
- Identify (A), (B), (C ),
Text Solution
|
- Convert
Text Solution
|
- Complete the following: b.
Text Solution
|
- Certain cyclic 1, 3-diketone give, under Clemmensen reduction, a fully...
Text Solution
|
Text Solution
|
Text Solution
|
Text Solution
|
Text Solution
|