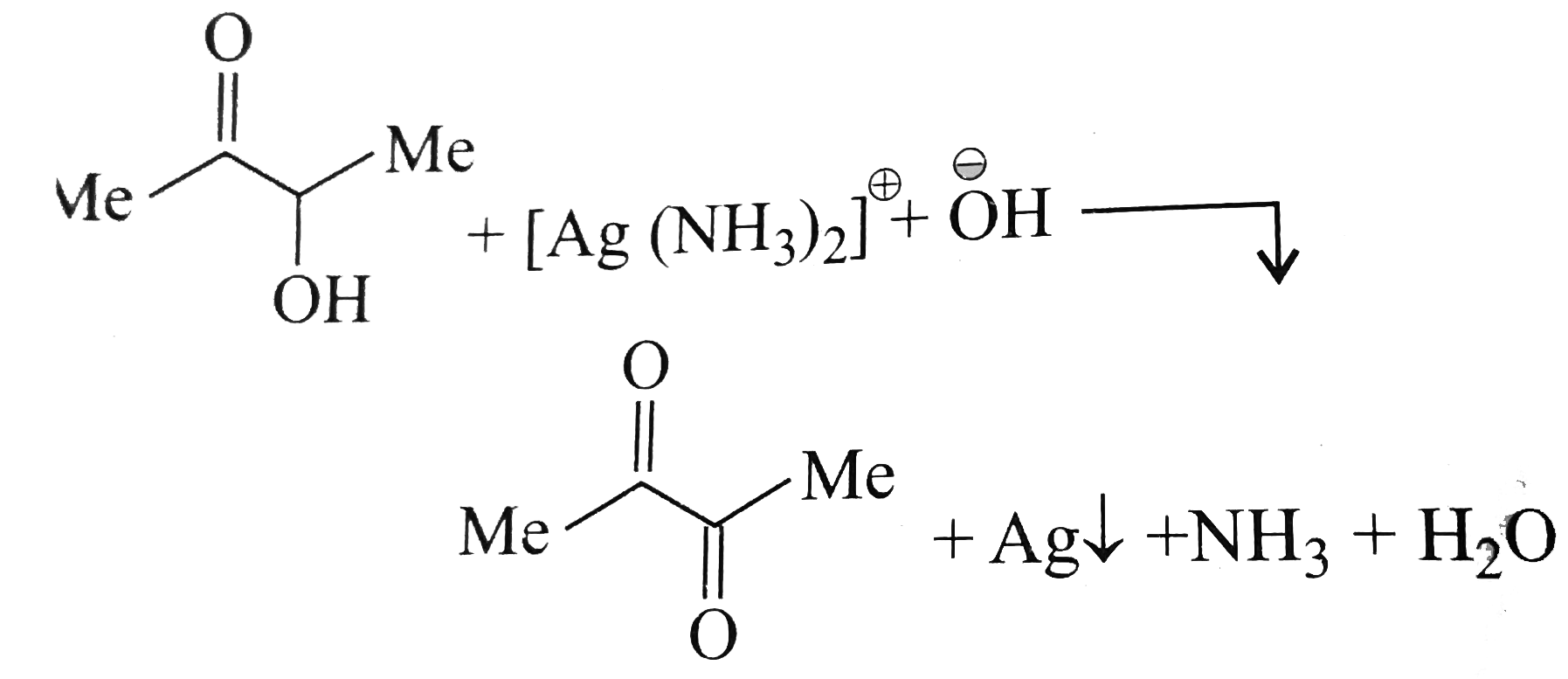
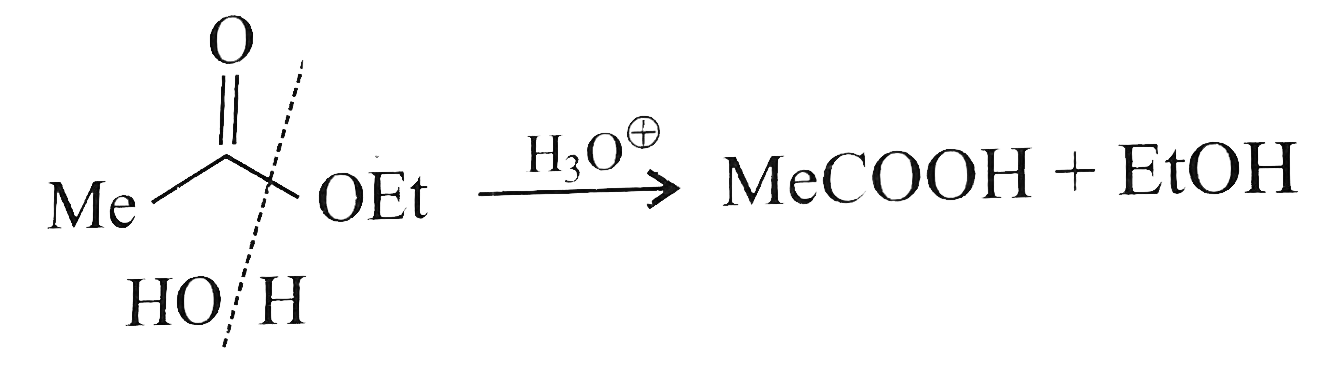
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Exercise (Single Correct)|90 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Exercise (Assertion And Reasoning)|10 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Exercise (Linked Comprehension)|52 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY|Exercise Viva Voce Questions And Part-C (Analysis Of Cations)|42 VideosSOLID STATE
CENGAGE CHEMISTRY|Exercise Ex 1.2 (Objective)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS-Exercise (Multiple Correct)
- In Q. No. 3, direct conversion of II to V can be carried by:
Text Solution
|
- Consider the following reactions: Which of the following group(s)...
Text Solution
|
- Compound C(4)H(8)O(2) exists in various strutures as shown: Whic...
Text Solution
|
- Consider the following Baeyer-Villiger oxidation.
Text Solution
|
- Select the correct Baeyer-Villiger oxidation reaction:
Text Solution
|
- Select the correct group(s) of reagent(s) used in the following conver...
Text Solution
|
- Which statements(s) is//are correct:
Text Solution
|
- Which of the following statement(s) is/are correct?
Text Solution
|
- In Q.No. 11, which of the following reagents can be used to convert (A...
Text Solution
|
- Which of the following will give yellow precipitate with KOI?
Text Solution
|
- Which of the following will undergo periodic oxidation?
Text Solution
|
- Which of the following reactions is//are correct?
Text Solution
|
- Which of the following reactions is//are correct?
Text Solution
|
- Which statement(s) is/are wrong?
Text Solution
|
- Which of the following reaction(s) is/are correct?
Text Solution
|
- Which of the following reaction(s) is/are wrong?
Text Solution
|
- Which of the following reactions is/are wrong?
Text Solution
|
- Which of the following unbalanced reactions is/are correct?
Text Solution
|
- Which of the following methods is/are correct for the synthesis of ben...
Text Solution
|
- Which of the following reaction is/are correct?
Text Solution
|