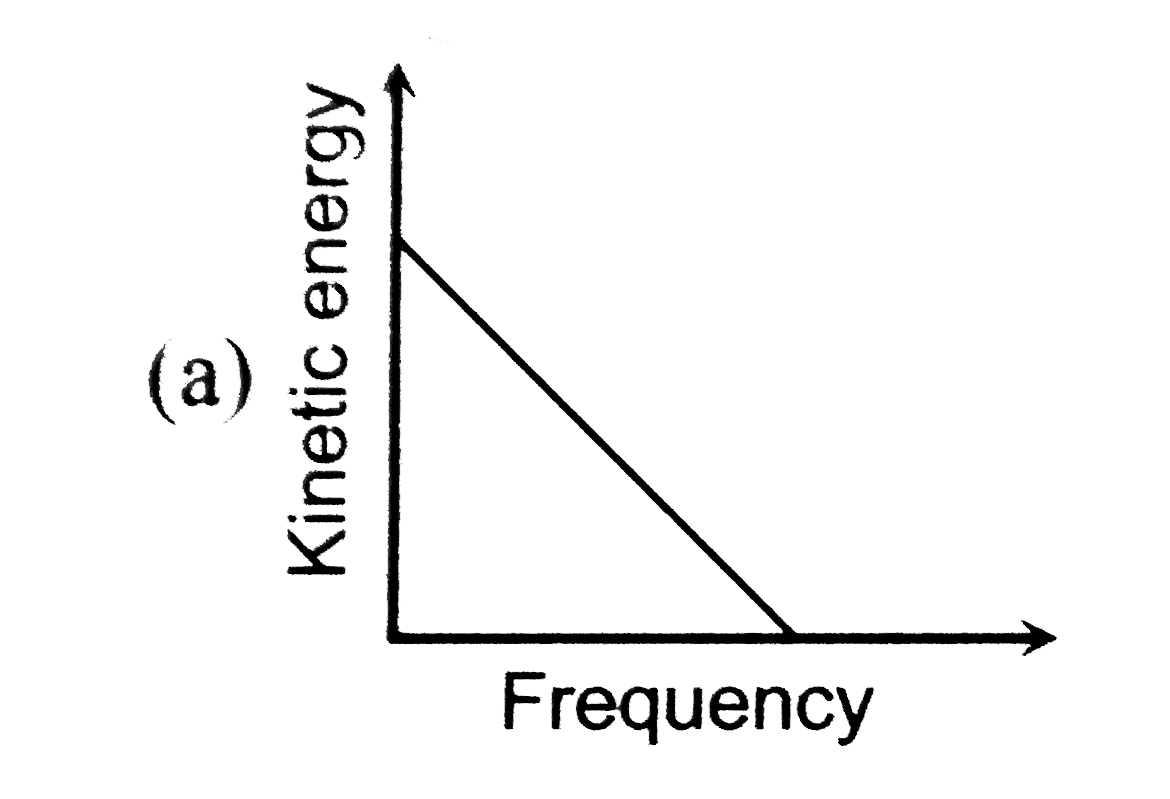
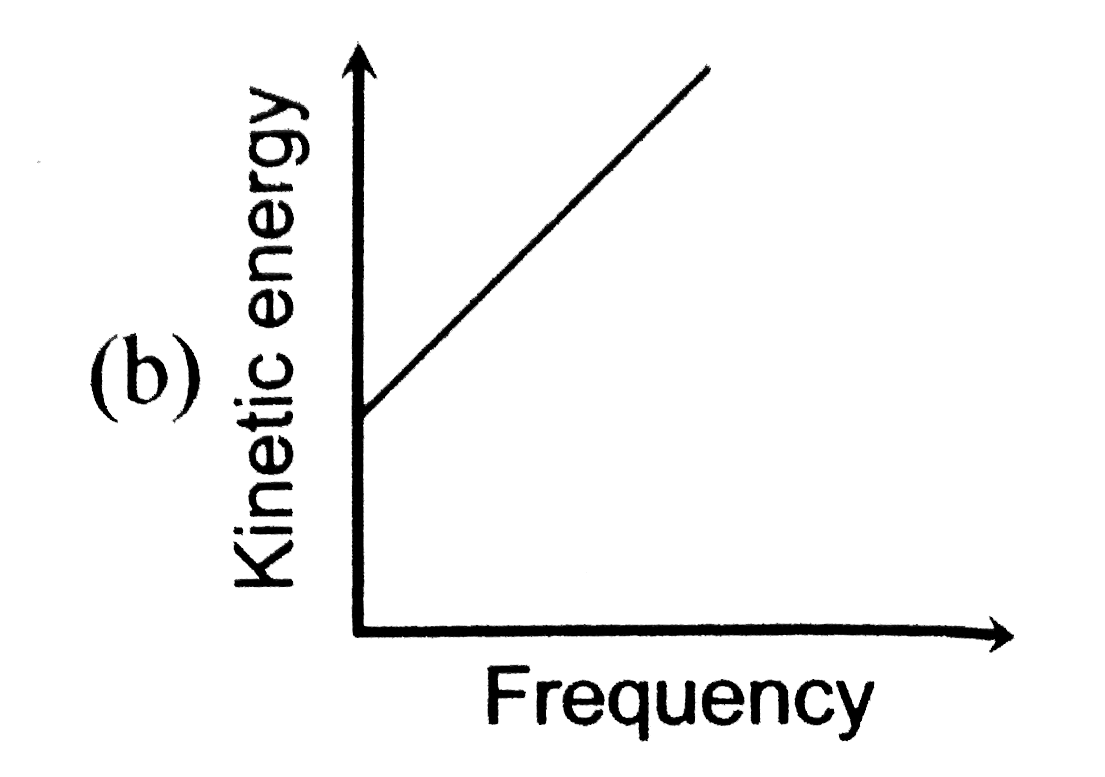
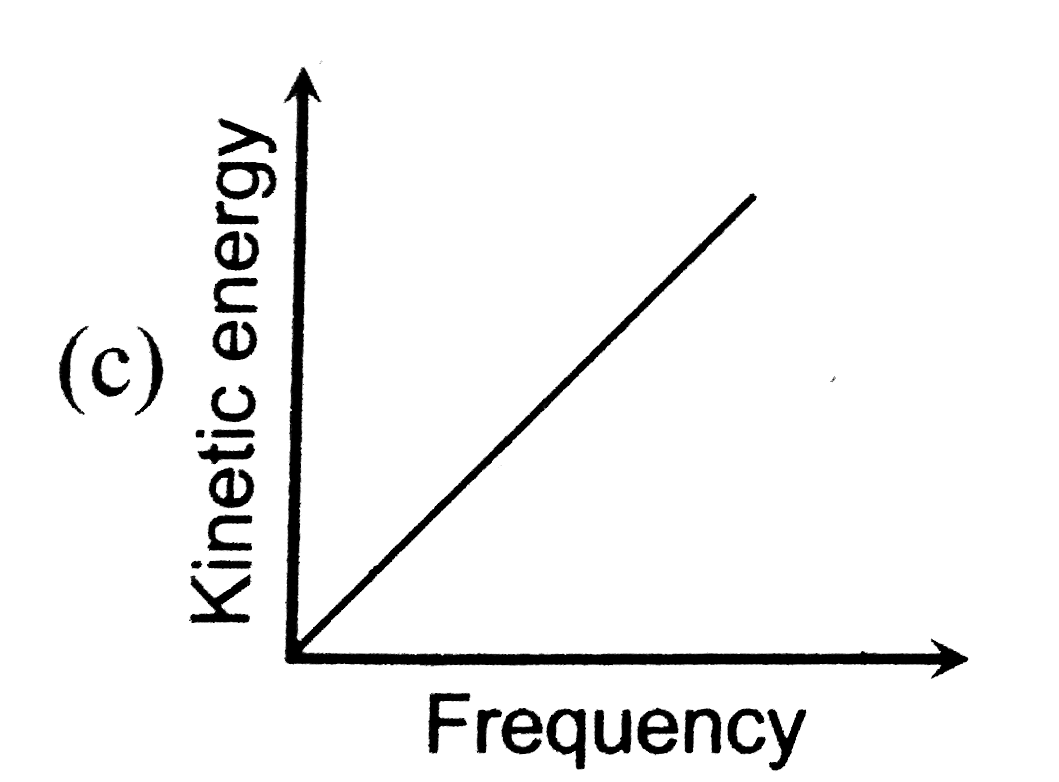
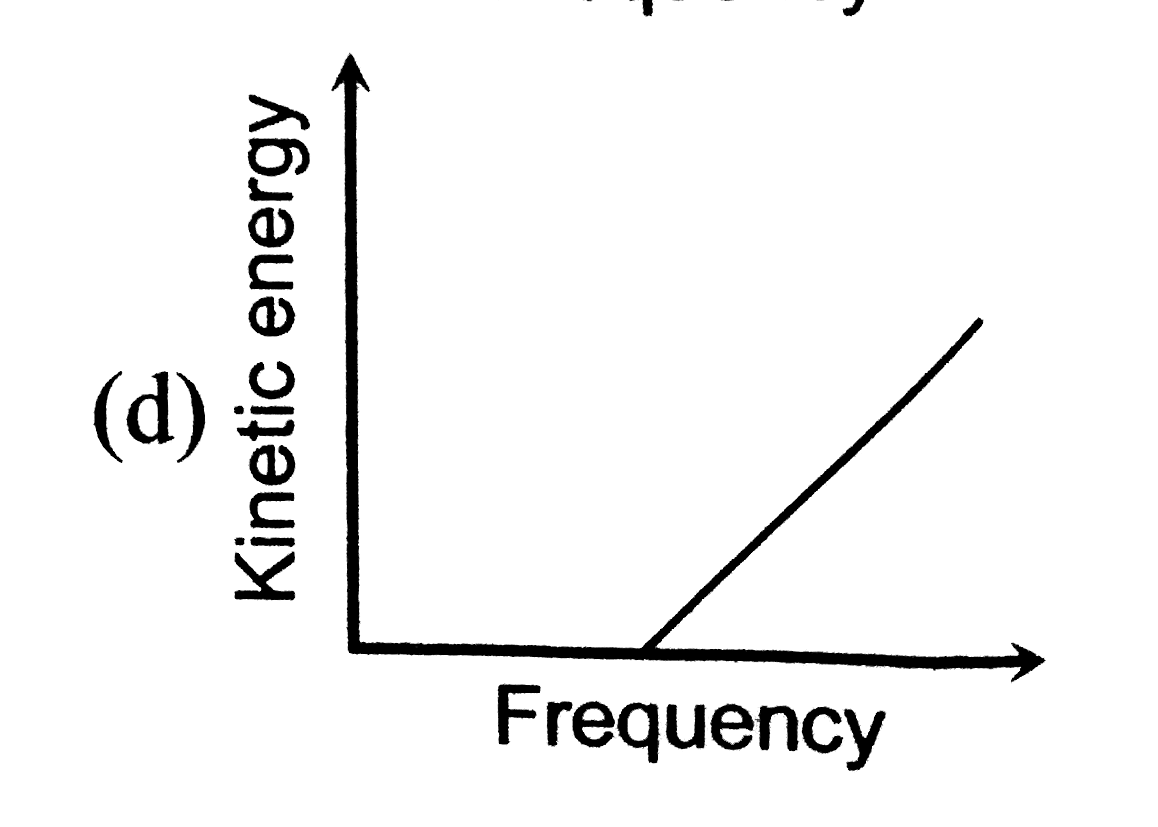
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
DUAL NATURE OF RADIATION AND MATTER
A2Z|Exercise X-Rays|45 VideosDUAL NATURE OF RADIATION AND MATTER
A2Z|Exercise Problems Based On Mixed Concepts|42 VideosDUAL NATURE OF RADIATION AND MATTER
A2Z|Exercise Photo Momentum Energy|30 VideosCURRENT ELECTRICITY
A2Z|Exercise Section D - Chapter End Test|29 VideosELECTRIC CHARGE, FIELD & FLUX
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-DUAL NATURE OF RADIATION AND MATTER-Photo Electric Effect
- If in a photoelectric experiment , the wavelength of incident radiatio...
Text Solution
|
- The photoelectric work function for a metal surface is 4.125 eV. The c...
Text Solution
|
- According to Einstein's photoelectric equation, the graph between the ...
Text Solution
|
- The stopping potential V for photoelectric emission from a metal surfa...
Text Solution
|
- In an experiment on photoelectric effect the frequency f of the incide...
Text Solution
|
- The work function of a metal is 1.6 xx 10^(-19) J. When the metal surf...
Text Solution
|
- Ultraviolet radiation of 6.2 eV falls on an aluminium surface (work -...
Text Solution
|
- The work function for tungsten and sodium are 4.5 eV and 2.3 eV respec...
Text Solution
|
- The work function of a metallic surface is 5.01 eV. The photo - electr...
Text Solution
|
- Light of wavelength 4000 Å falls on a photosensitive metal and a negat...
Text Solution
|
- When yellow light is incident on a surface , no electrons are emitted ...
Text Solution
|
- The photoelectric threshold of a certain metal is 3000A. If the radiat...
Text Solution
|
- A photocell stoops emission if it is maintained at 2 V negative potent...
Text Solution
|
- Assuming photoemission to take place , the factor by which the maximum...
Text Solution
|
- When a point source of light is at a distance of one metre from a phot...
Text Solution
|
- If the work function of a metal is 'phi' and the frequency of the inci...
Text Solution
|
- Light of wavelength lambda strikes a photo - sensitive surface and ele...
Text Solution
|
- Light of wavelength 5000 Å falls on a sensitive plate with photoelectr...
Text Solution
|
- If the work function of a photo - metal is 6.825 eV. Its threshold wav...
Text Solution
|
- Work function of a metal is 2.1 eV. Which of the waves of the followin...
Text Solution
|