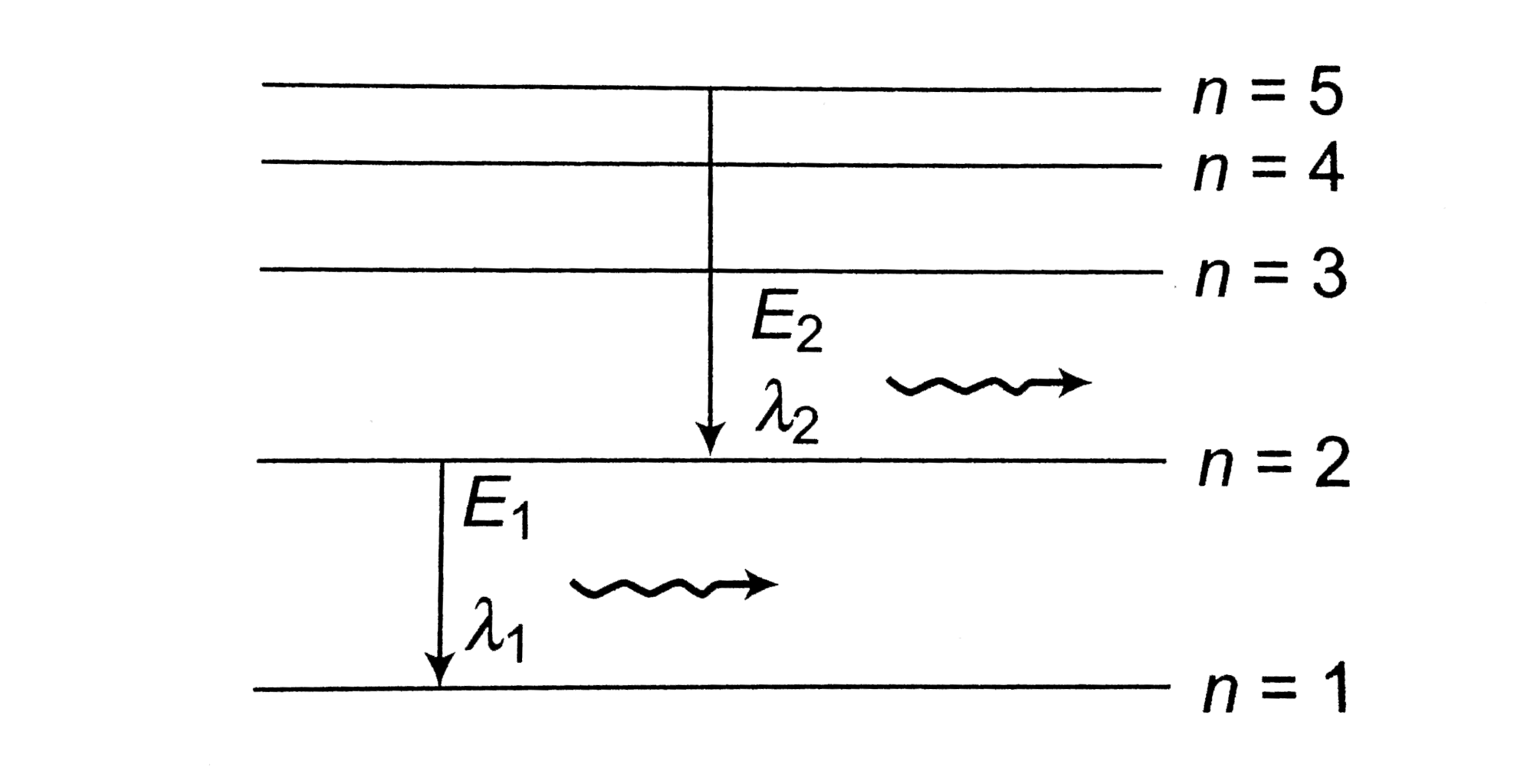A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
A2Z|Exercise Atomic Spectrum|53 VideosATOMIC PHYSICS
A2Z|Exercise Problems Based On Mixed Concepts|43 VideosATOMIC PHYSICS
A2Z|Exercise Section D - Chapter End Test|30 VideosALTERNATING CURRENT
A2Z|Exercise Section D - Chapter End Test|30 VideosCURRENT ELECTRICITY
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-ATOMIC PHYSICS-Bohr'S Hydrogen Model
- The wavelength of light emitted from second orbit to first orbits in a...
Text Solution
|
- Energy of the electron in nth orbit of hydrogen atom is given by E(n) ...
Text Solution
|
- The de-Broglie wavelength of an electron in the first Bohr orbit is
Text Solution
|
- In hydrogen atom, when electron jupms from second to first orbit, then...
Text Solution
|
- Minimum energy required to takeout the only one electron from ground s...
Text Solution
|
- The frequency of 1st line Balmer series in H(2) atom is v(0). The freq...
Text Solution
|
- When the electron in the hydrogen atom jumps from 2nd orbit to 1st orb...
Text Solution
|
- Which of the following transitions will have highest emission waveleng...
Text Solution
|
- When the wave of hydrogen atom comes from infinity into the first then...
Text Solution
|
- With the increase in peinciple quantum number, the energy difference b...
Text Solution
|
- In which of the following systems will the radius of the first orbit (...
Text Solution
|
- If the binding energy of the electron in a hydrogen atom is 13.6 eV, t...
Text Solution
|
- Energy E of a hydrogen atom with principle quantum number n is given b...
Text Solution
|
- Which state of triply ionised Beryllium (Be^(+++)) the same orbital ra...
Text Solution
|
- The ratio of areas within the electron orbits for the first excited st...
Text Solution
|
- The kinetic energy of electron in the first Bohr orbit of the hydrogen...
Text Solution
|
- If the energy of a hydrogen atom in nth orbit is E(n), then energy in ...
Text Solution
|
- What is the ratio of wavelength of radiations emitted when an electron...
Text Solution
|
- The ground state energy of hydrogen atom is -13.6 eV. What is the pote...
Text Solution
|
- The diagram shown the energy levels for an electron in a certain atom....
Text Solution
|