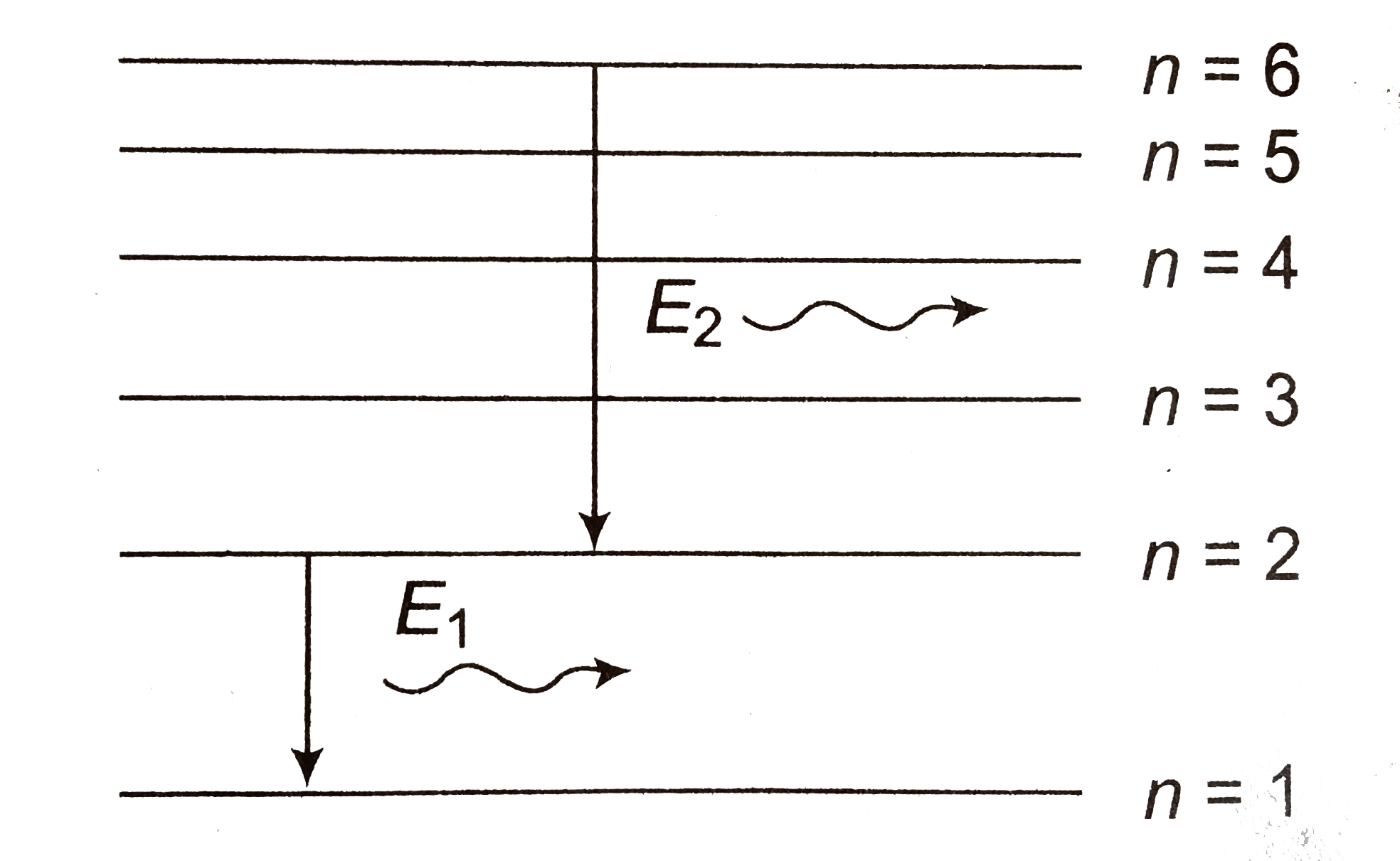
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
A2Z|Exercise Assertion Reason|5 VideosATOMIC PHYSICS
A2Z|Exercise Section D - Chapter End Test|30 VideosATOMIC PHYSICS
A2Z|Exercise AIPMT/NEET Questions|31 VideosALTERNATING CURRENT
A2Z|Exercise Section D - Chapter End Test|30 VideosCURRENT ELECTRICITY
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-ATOMIC PHYSICS-AIIMS Questions
- A neutron makes a head-on elastic collision with a stationary deuteron...
Text Solution
|
- The ground state energy of hydrogen atom is -13.6 eV. What is the pote...
Text Solution
|
- Solid targets of different elements are bombarded by highly energetic ...
Text Solution
|
- Hydrogen atom emits blue light when it changes from n = 4 energy level...
Text Solution
|
- Energy of the electron in nth orbit of hydrogen atom is given by E(n) ...
Text Solution
|
- An electron changes its position from orbit n = 4 to the orbit n = 2 o...
Text Solution
|
- As the electron in the Bohr orbit is hydrogen atom passes from state n...
Text Solution
|
- Which of the following transitions in a hydrogen atom emits photon of ...
Text Solution
|
- How much work must be done to pull apart the electron and the proton t...
Text Solution
|
- Energy levels A, B, C of a certain atom corresponding to increasing va...
Text Solution
|