Text Solution
Verified by Experts
Topper's Solved these Questions
BEHAVIOUR OF PERFECT GAS & KINETIC THEORY
PRADEEP|Exercise Conceptual Problems|23 VideosBEHAVIOUR OF PERFECT GAS & KINETIC THEORY
PRADEEP|Exercise Very short Answer questions|35 VideosBEHAVIOUR OF PERFECT GAS & KINETIC THEORY
PRADEEP|Exercise Curiosity Question|2 VideosGRAVIATION
PRADEEP|Exercise Assertion-Reason Type Questions|19 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-BEHAVIOUR OF PERFECT GAS & KINETIC THEORY-Solved Examples
- Molar volume is the volume occupied by 1 mole of any (Ideal) gas at st...
Text Solution
|
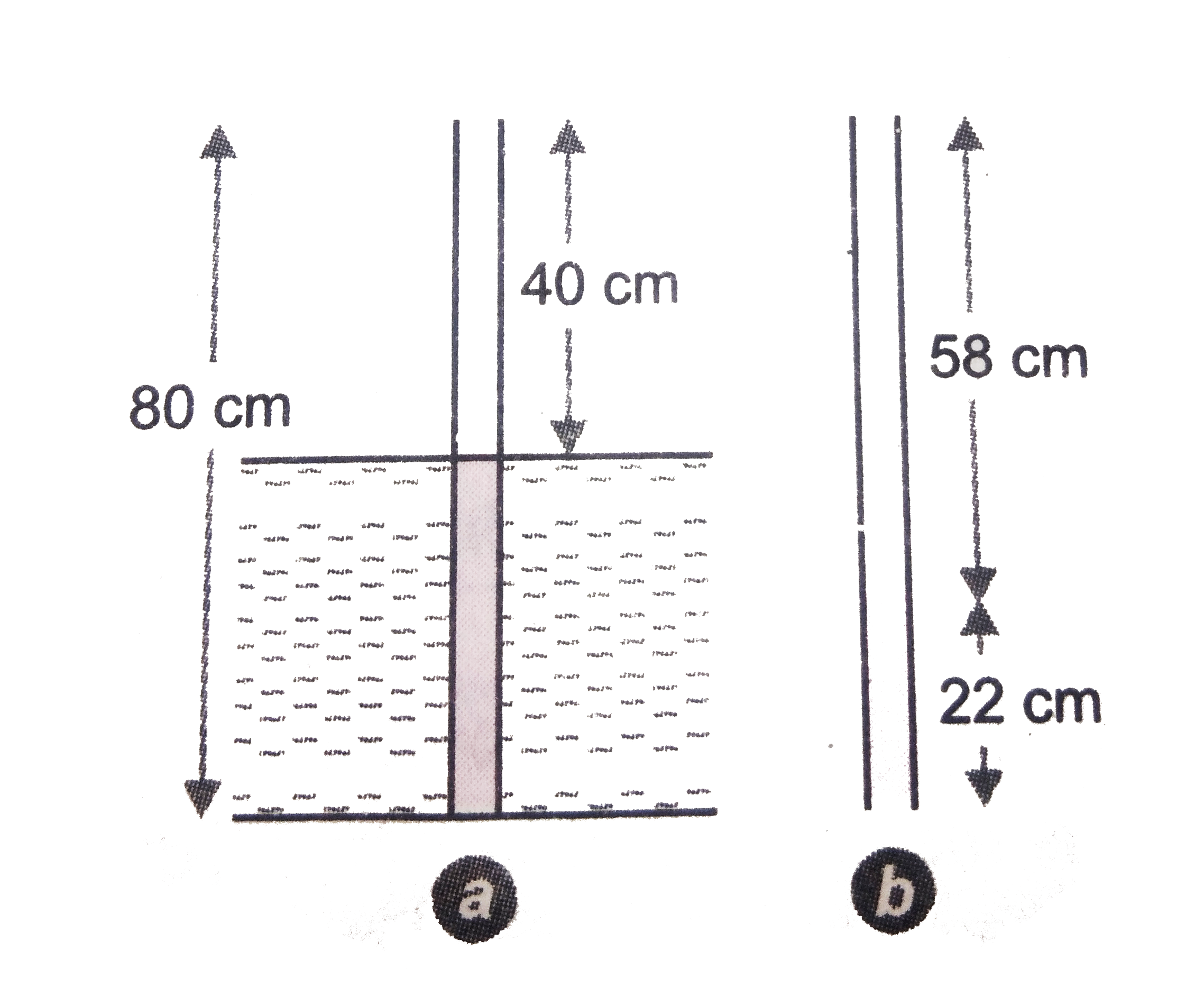
- A narrow uniform glass tube 80 cm long and open at both ends is half i...
Text Solution
|
- The density of water is 1000 kg m^(-3). The density of water vapour at...
Text Solution
|
- Estimate the volume of a water molecule using the data in the above qu...
Text Solution
|
- What is the average distance between atoms (interatomic distance) in w...
Text Solution
|
- A gas at 27^@ C in a cylinder has a volume of 4 litre and pressure 100...
Text Solution
|
- A closed container of volume 0.02m^3contains a mixture of neon and arg...
Text Solution
|
- A narrow uniform glass tube contains air enclosed by 15 cm long thread...
Text Solution
|
- A vessel contains two non-reactive gases neon (monoatomic) and oxygen ...
Text Solution
|
- Two soap bubbles of radii a and b coalesce to form a single bubble of ...
Text Solution
|
- If the temperature of air is increased from 27^(@) to 227^(@), in what...
Text Solution
|
- Calculate for hydrogen at 27^(@) (i) KE of one gram mole of the gas ...
Text Solution
|
- A vessel is filled with a gas at a pressure of 76 cm of mercury at a c...
Text Solution
|
- Calculate the number of molecules in 2 xx 10^(-6)m^(3) of a perfect ga...
Text Solution
|
- Two perfect gases at absolute temperature T(1) and T(2) are mixed. The...
Text Solution
|
- Kinetic energy of oxygen molecule at 0^(@) C is 9.4 xx 10^(-21) J. Cal...
Text Solution
|
- Calculate (i) rms velocity and (ii) mean kinetic energy of one gram mo...
Text Solution
|
- A flask contains argon and chlorine in the ratio 2:1 by mass. The temp...
Text Solution
|
- Uranium has two isotopes of masses 235 and 238 units. If both are pres...
Text Solution
|
- (a) When a molecule (or an elastic ball) hits a (massive) wall, it reb...
Text Solution
|