Text Solution
Verified by Experts
Topper's Solved these Questions
BEHAVIOUR OF PERFECT GAS & KINETIC THEORY
PRADEEP|Exercise Very short Answer questions|35 VideosBEHAVIOUR OF PERFECT GAS & KINETIC THEORY
PRADEEP|Exercise Short answer questions|28 VideosBEHAVIOUR OF PERFECT GAS & KINETIC THEORY
PRADEEP|Exercise Solved Examples|43 VideosGRAVIATION
PRADEEP|Exercise Assertion-Reason Type Questions|19 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-BEHAVIOUR OF PERFECT GAS & KINETIC THEORY-Conceptual Problems
- Under what conditions do the real gases obey more strictly the gas equ...
Text Solution
|
- Two perfect gases at absolute temperature T(1) and T(2) are mixed. The...
Text Solution
|
- A box contains equal number of molecules of hydrogen and oxygen. If th...
Text Solution
|
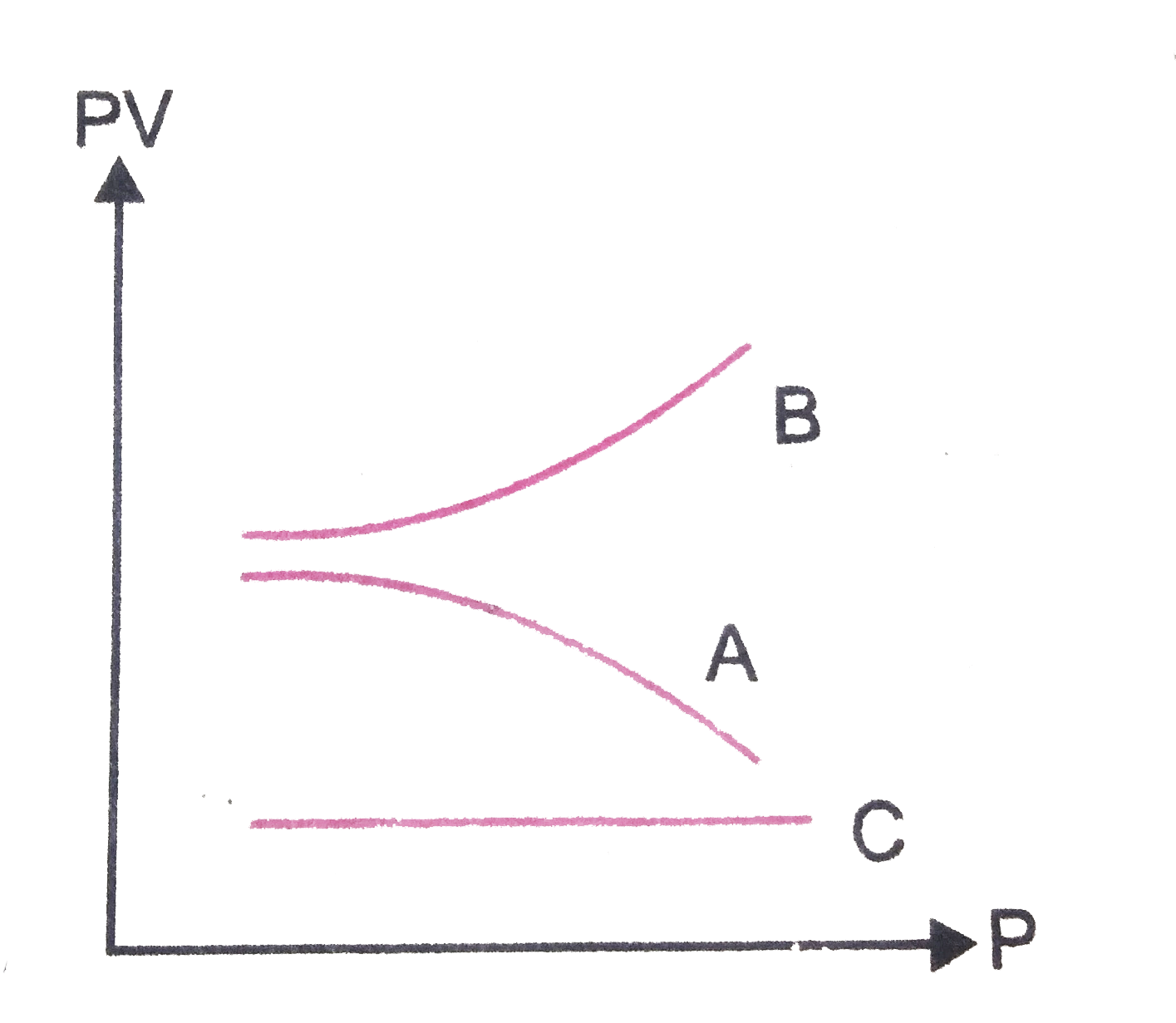
- The graphs in Fig shown the variation of the product PV with respect t...
Text Solution
|
- Two different gases at the same temperature have equal root mean squar...
Text Solution
|
- Distinguish between average speed and rms speed. If three molecules ha...
Text Solution
|
- A gas is filled in a cylinder fitted with a piston at a definite tempe...
Text Solution
|
- A gas is filled in a cylinder fitted with a piston at a definite tempe...
Text Solution
|
- On reducing the volume of the gas at constant temperature, the pressur...
Text Solution
|
- Explain the phenomenon of evaporation on the basis of kinetic theory.
Text Solution
|
- Why cooling is caused by evaporation?
Text Solution
|
- The rms speed of oxygen molecules at a certain temperature T is v. If ...
Text Solution
|
- A vessel of volume 4 litres contains a mixture of 8 grams of O(2), 14 ...
Text Solution
|
- Why temperature less than OK is not possible ?
Text Solution
|
- The ratio of vapour densities of two gases at the same temperature is ...
Text Solution
|
- How can the number of molecular collisions per unit time in a gas be i...
Text Solution
|
- On which factors does the average KE of gas molecules depend : nature ...
Text Solution
|
- What happens when a compressed gas pushes a piston out and expands ?
Text Solution
|
- Though the velocity of air molecules is nearly 0.5 km//s, yet the smel...
Text Solution
|
- There are N molecules of a gas in a containter. If this number is incr...
Text Solution
|