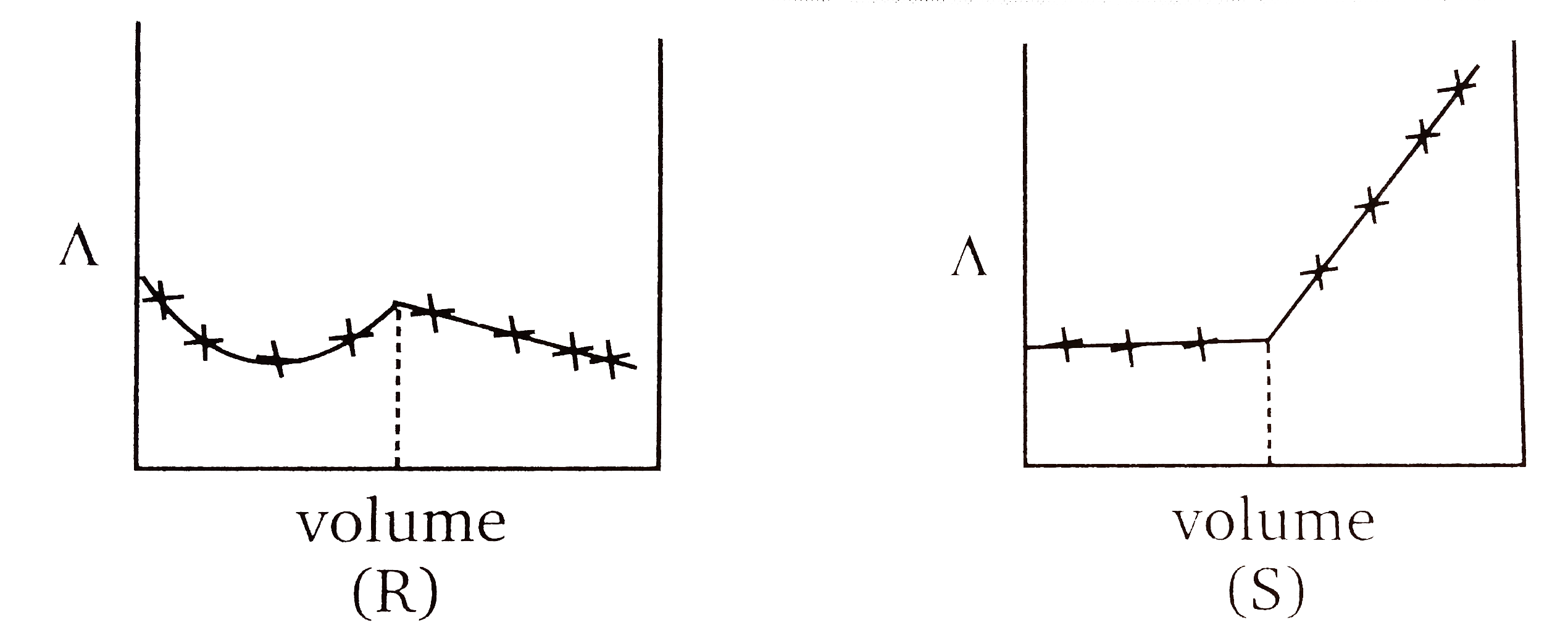
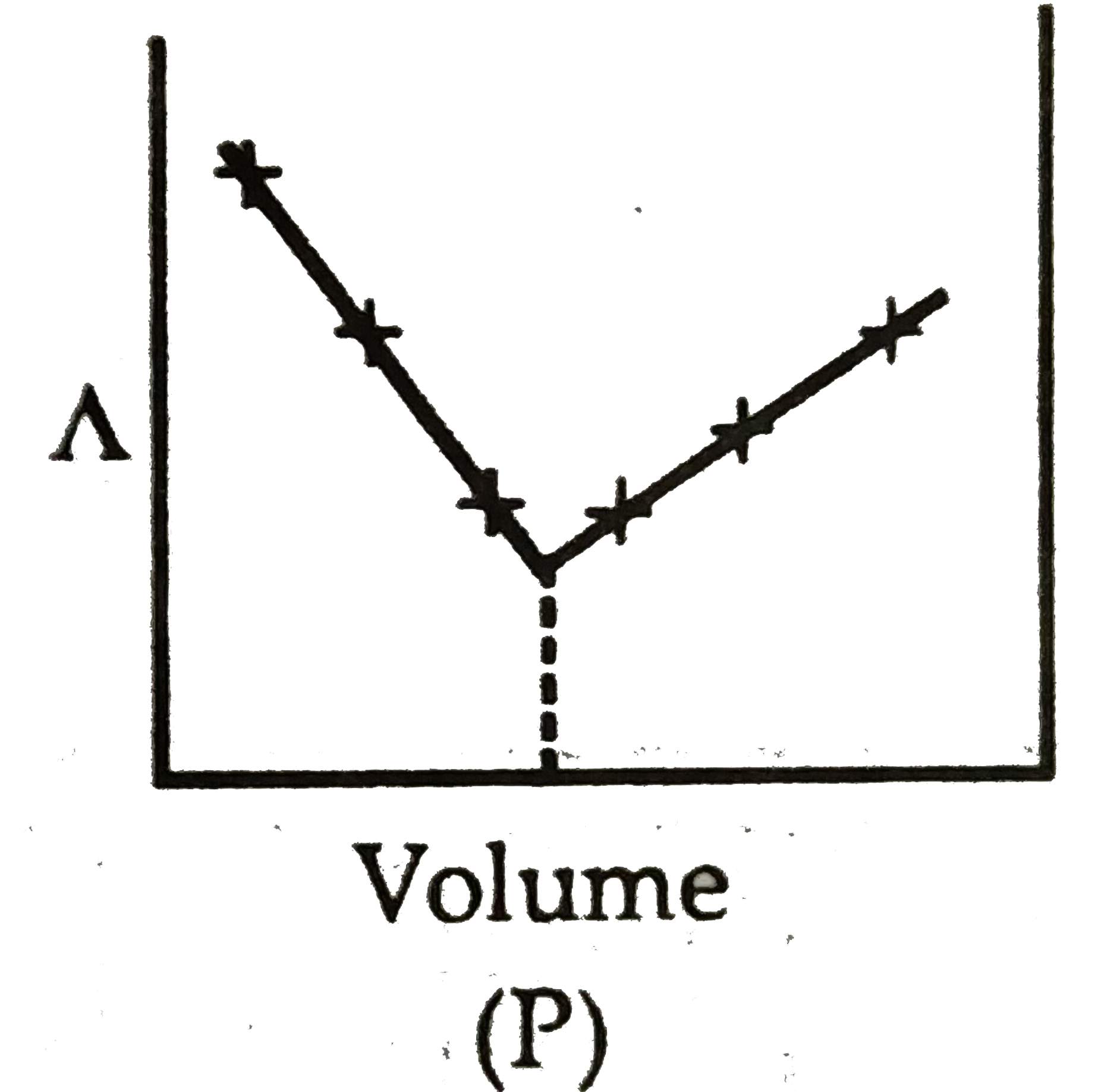
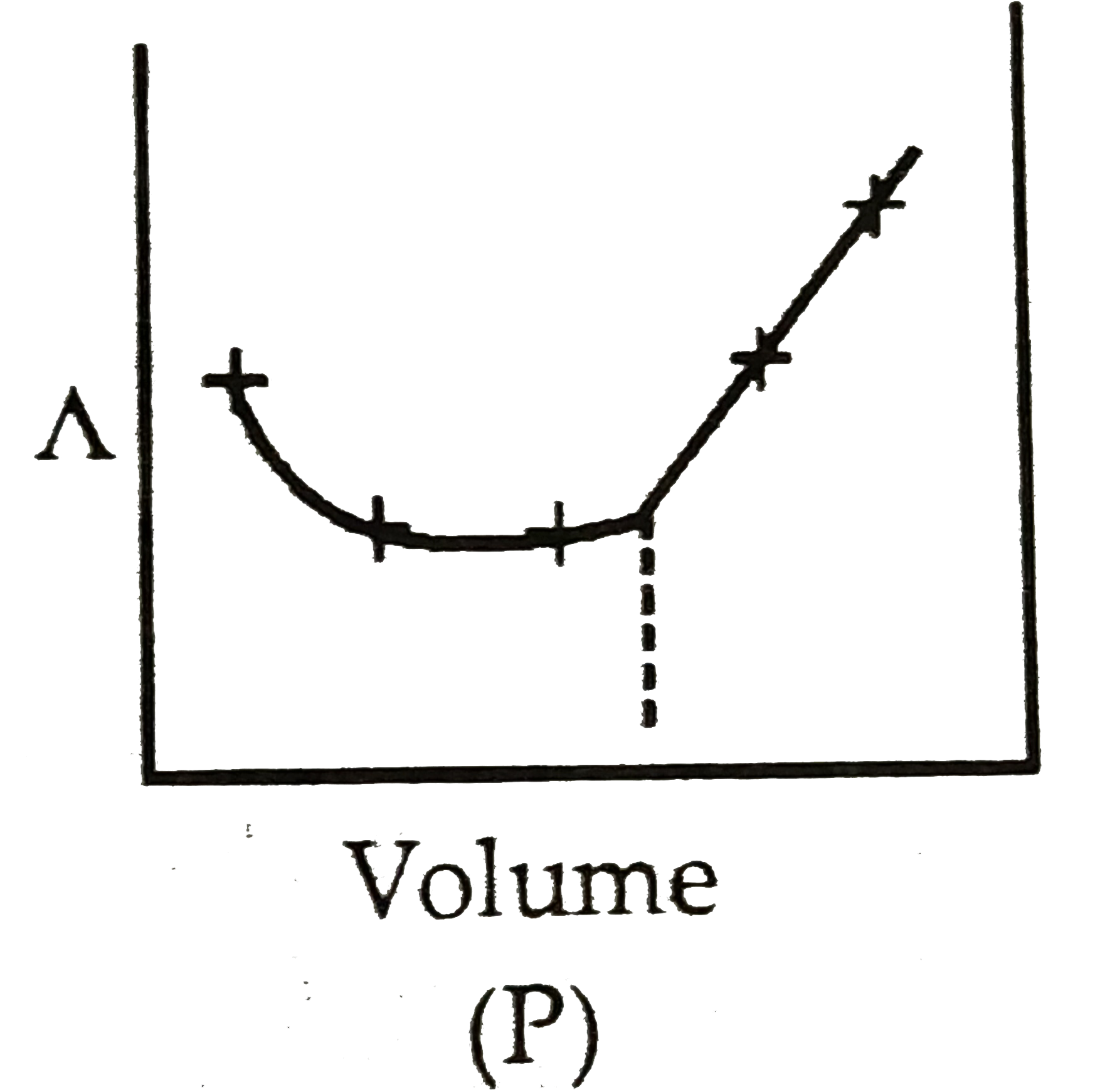
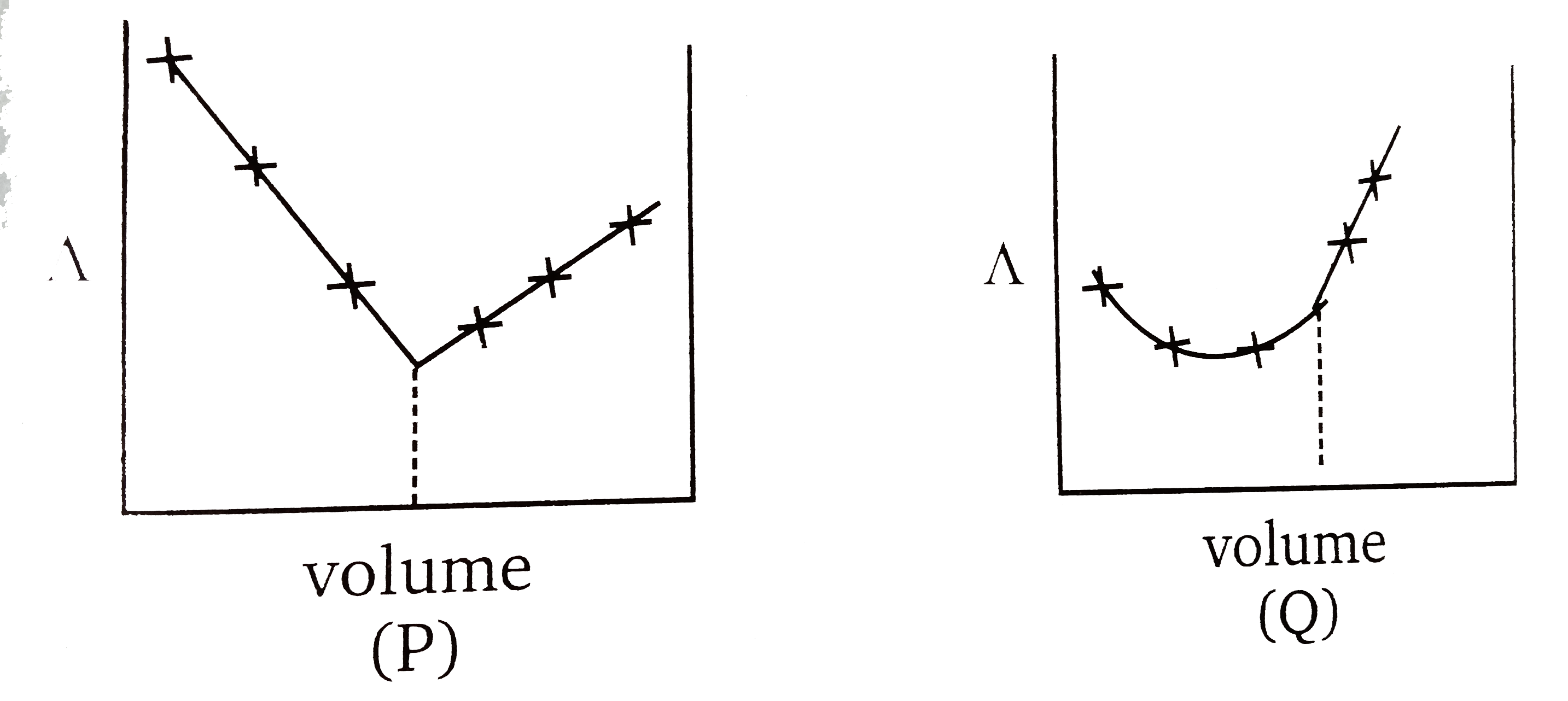A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NIKITA PUBLICATION-ELECTROCHEMISTRY -Questions From Competitive Exam
- The reduction potential of hydrogen half cell will be negative if :
Text Solution
|
- Resistance of 0.2 M solution of an electrolyte is 50 Omega. The specif...
Text Solution
|
- AgNO(3)(aq.) was added to an aqeous KCl solution gradually and the con...
Text Solution
|
- Consider the following cell reaction. 2Fe(s)+O(2)(g)+4H^(+)(aq)rarr2...
Text Solution
|
- A solution contains Fe^(2+), Fe^(3+) and T^(-) ions. This solution was...
Text Solution
|
- The ionic conductance of Ba^(2+) and Cl^(-) respectively are 127 and 7...
Text Solution
|
- What volume of O(2) at NTP liberated by 5 A current flowing for 193 a...
Text Solution
|
- Find E(cell)^(@) for the cell : Zn | Zn^(2+)(1M) || Ag^(+)(1M) | Ag ...
Text Solution
|
- The number of moles of electrone required to deposits 36 g of Al from ...
Text Solution
|
- The emf in V of Danneil cell containing 0.1 M ZnSO(4) and 0.01 M CuSO(...
Text Solution
|
- Cell constant of a conductivity cell is ?
Text Solution
|
- The standard reduction potential for Zn^(2+)//Zn, Ni^(2+)//Ni and Fe^(...
Text Solution
|
- The solubility product (K(sp) , mol^(3) dm^(-9) ) of MX(2) at 298 K ba...
Text Solution
|
- The value of Delta G (kJ mol| -1) for the given cell is (take 1 F = 96...
Text Solution
|
- Al(2)O(3) is reduced by electrolysis at low potentials and high curren...
Text Solution
|
- The equivalent conductance of M//32 solution of a weak monobasic acid ...
Text Solution
|
- The ionic conductance is least for
Text Solution
|
- Given, standard electrode potentials Fe^(2+) +2e^(-) rarr Fe, E^(@)=...
Text Solution
|
- Electrode potential of hydrogen electrode is 18 m V then [H^(+)] is -
Text Solution
|
- A certain quantity of electricity is passed through aq. Al(2)(SO(4))(3...
Text Solution
|