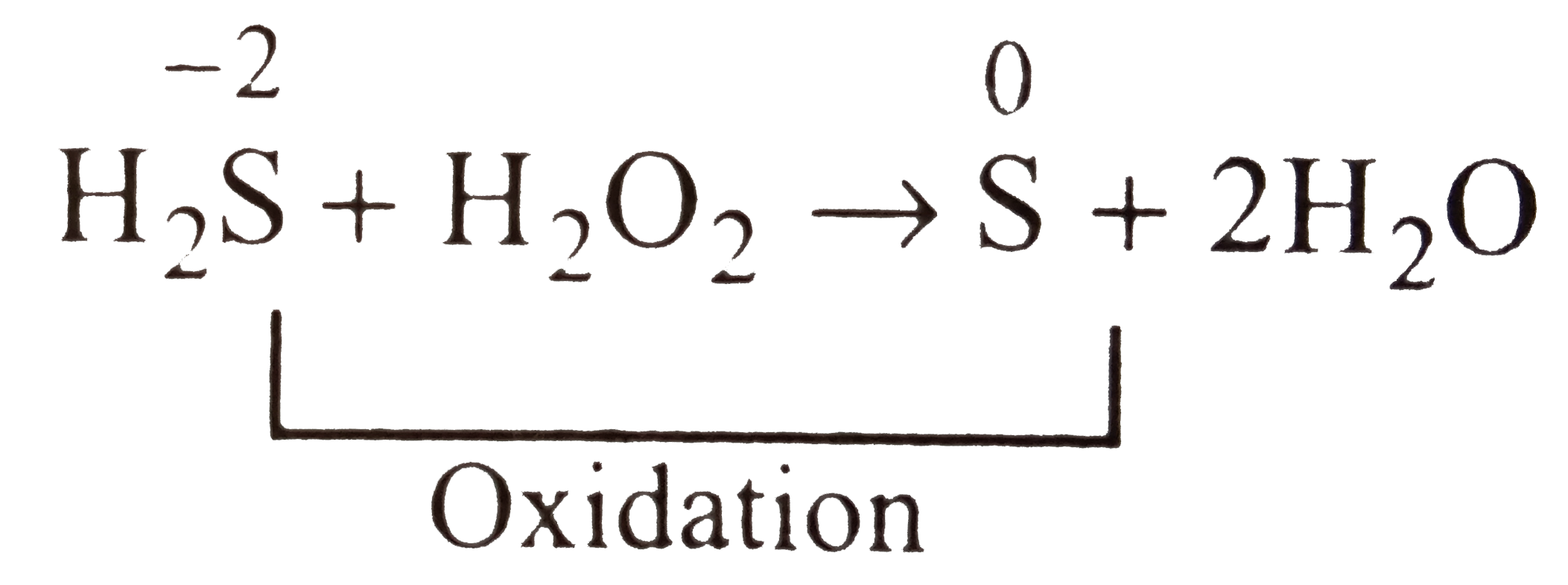A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
A2Z|Exercise Balancing Of The Equation|28 VideosREDOX REACTIONS
A2Z|Exercise Stoichiometry In Redox Reactions|44 VideosP BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise Section D - Chapter End Test|30 VideosS BLOCK ELEMENTS ( GROUP 13 - 14)
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-REDOX REACTIONS-Section D - Chapter End Test
- Equation H(2)S+H(2)O(2) rarr S+2H(2)O represents
Text Solution
|
- For H(3)PO(3) and H(3)PO(4) the correct choice is
Text Solution
|
- The oxidation number of sulphur in H(2)S(2)O(7) and iron in K(4)Fe(CN)...
Text Solution
|
- One mole of N(2)H(4) loses ten moles of electrons to form a new compou...
Text Solution
|
- In the compound YBa(2)Cu(3)O(7) which shows superconductivity, what is...
Text Solution
|
- The oxidation number of S in S(8),S(2)F(2), and H(2)S, respectively, a...
Text Solution
|
- Which one of the following reactions is not an example of redox reacti...
Text Solution
|
- For the reaction, C+O(2)rarrCO(2), DeltaH=-393 J 2Zn+O(2) rarr 2ZnO,...
Text Solution
|
- In the reaction B(2)H(6)+2KOH+2Xrarr 2Y+6H(2), X and Y are respectivel...
Text Solution
|
- In a balanced equation H(2)SO(4)+xHI rarr H(2)S+YI(2)+zH(2)O, the valu...
Text Solution
|
- MnO(4)^(2-) (1 mole) in neutral aqueous medium is disproportionate to
Text Solution
|
- The conductivity of a saturated solution of BaSO4 is 3. 06 xx 10^(-6)...
Text Solution
|
- H(2)O(2) reduces K(4)Fe(CN)(6)
Text Solution
|
- When sodium metal is dissolved in liquid ammonia, blue colour solution...
Text Solution
|
- Which of the following is redox reaction ?
Text Solution
|
- In which of the following reactions H(2)O(2) is a reducing agent?
Text Solution
|
- Which is the best description of the behaviour of bromine in the react...
Text Solution
|
- Which of the following substances acts as an oxidising as well as a re...
Text Solution
|
- When K(2)Cr(2)O(7) is converted to K(2)CrO(4), the change in the oxida...
Text Solution
|
- Oxidation state of chlorine in perchloric acid is
Text Solution
|
- The oxidation number of S in H(2)S(2)O(8) is
Text Solution
|