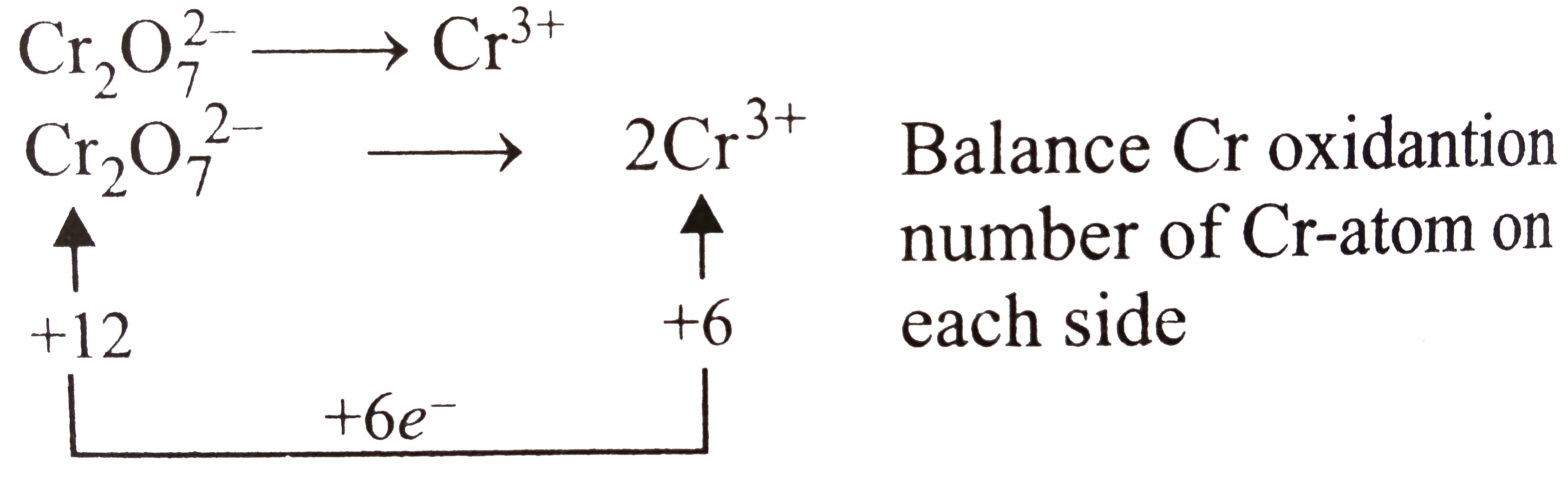A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
A2Z|Exercise Section B - Assertion Reasoning|25 VideosREDOX REACTIONS
A2Z|Exercise AIPMT/ NEET Questions|16 VideosREDOX REACTIONS
A2Z|Exercise Stoichiometry In Redox Reactions|44 VideosP BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise Section D - Chapter End Test|30 VideosS BLOCK ELEMENTS ( GROUP 13 - 14)
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-REDOX REACTIONS-Type Of Redox Reaction And Equivalent Weight
- What volume of O(2) measured at standard condition will be formed by t...
Text Solution
|
- The equivalent weight of MnSO(4) is half its molecular weight when it ...
Text Solution
|
- Equivalent weight of K(2)Cr(2)O(7) in the following reaction is Cr(2...
Text Solution
|
- Which of the following reaction is a redox reaction?
Text Solution
|
- In the equation H(2)S+2HNO(3) rarr 2H(2)O+2NO(2)+S The equivalent weig...
Text Solution
|
- In the following reaction, 2H(2)S(g)+SO(2)(g) rarr 3S(s)+2H(2)O(l) ...
Text Solution
|
- 0.05 moles of NaHCO(3) will react with how many equivalent of Mg(OH)(2...
Text Solution
|
- Equivalent weight of S in SO(3)^(2-) is (S=32)
Text Solution
|
- The equivalent weight of MnSO(4) is half its molecular weight when it ...
Text Solution
|
- In the reaction, I(2)+2S(2)O(3)^(2-) rarr 2I^(-)+S(4)O(6)^(2-). Eq...
Text Solution
|
- Which has maximum number of equivalent per mole of the oxidant?
Text Solution
|
- The equivalent weight of Mohr's salt FeSO(4). (NH(4))(2)SO(4). 6H(2)O ...
Text Solution
|
- In alkaline medium , KMnO(4) reacts as follows 2KMnO(4)+2KOH rarr 2K...
Text Solution
|
- An element forms an oxide, in which the oxygen is 20% of the oxide by ...
Text Solution
|
- Photosynthesis of carbohydrates in plants takes place as 6CO(2)+12H(...
Text Solution
|
- The equivalent weight of phosphoric acid (H(3)PO(4)) in the reaction N...
Text Solution
|
- The equivalent weight of KIO(3) in the reaction 2Cr(OH)(3)+4OH+KIO(3) ...
Text Solution
|
- What is the equivalent weight of HNO(3) in the given reaction? 4Zn+1...
Text Solution
|
- In the following reaction (unbalanced), equivalent weight of As(2)S(3)...
Text Solution
|
- What is the equivalent weight of C(12)H(22)O(11) in the following reac...
Text Solution
|