A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-MOCK TEST-Mock Test 2
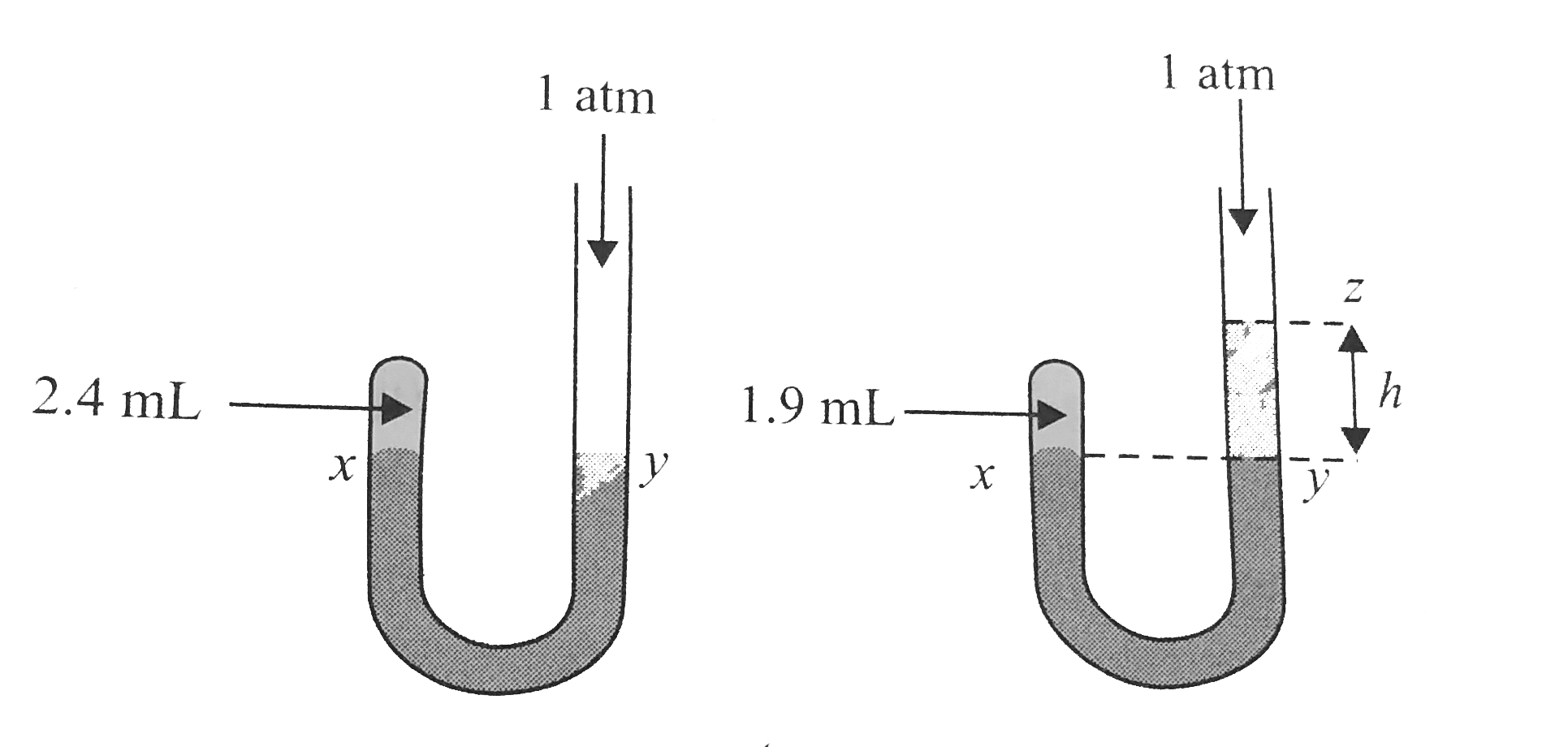
- Given J-tube has 2.4 mL of air at a pressure of 1 atm. On adding mercu...
Text Solution
|
- Ethylene combines with sulphur monochloride to form.
Text Solution
|
- Benzene reacts with acetyl chloride in the prescence of anhydrous AlCl...
Text Solution
|
- R-C -= C - H overset (NaNH2)rarr A overset (RX)rarr B overset (Na//Liq...
Text Solution
|
- 20 ml of an H2 O2 solution on reaction with excess of acidified KMnO4 ...
Text Solution
|
- Hydrogen diffuses six times faster than gas A. The molar mass of gas A...
Text Solution
|
- Which of the following forms a homologous series ?
Text Solution
|
- Which of the following alkanes cannot be produced by Kolbe's electroly...
Text Solution
|
- Which is not a tetrahedral species ?
Text Solution
|
- A mixture of CO, CO2 and He on passing over red hot coke shows 40 % in...
Text Solution
|
- A gas with negligible intermolecular interactions shows compressibilit...
Text Solution
|
Text Solution
|
- The conductivity of a saturated solution of BaSO4 is 3. 06 xx 10^(-6)...
Text Solution
|
- The reaction proceeds through .
Text Solution
|
- IE "for" He^+ "is" 1.96 xx 10^-19 J "atom"^-1. Calculate the energy of...
Text Solution
|
- Which of the following compounds will exhibit d-orbital resonance ?
Text Solution
|
- The heat of atomisation of PH(3(g)) is 228kcal mol^(-1) and that of P(...
Text Solution
|
- Which of the following pairs of compounds may be regarded both as posi...
Text Solution
|
- The correct decreasing order of ionic size among the following species...
Text Solution
|
- One mole of symmetrical alkene on ozonolysis gives two moles of an ald...
Text Solution
|
- The Gibbs energy for the decomposition of FeO at 600^@ C is as follows...
Text Solution
|