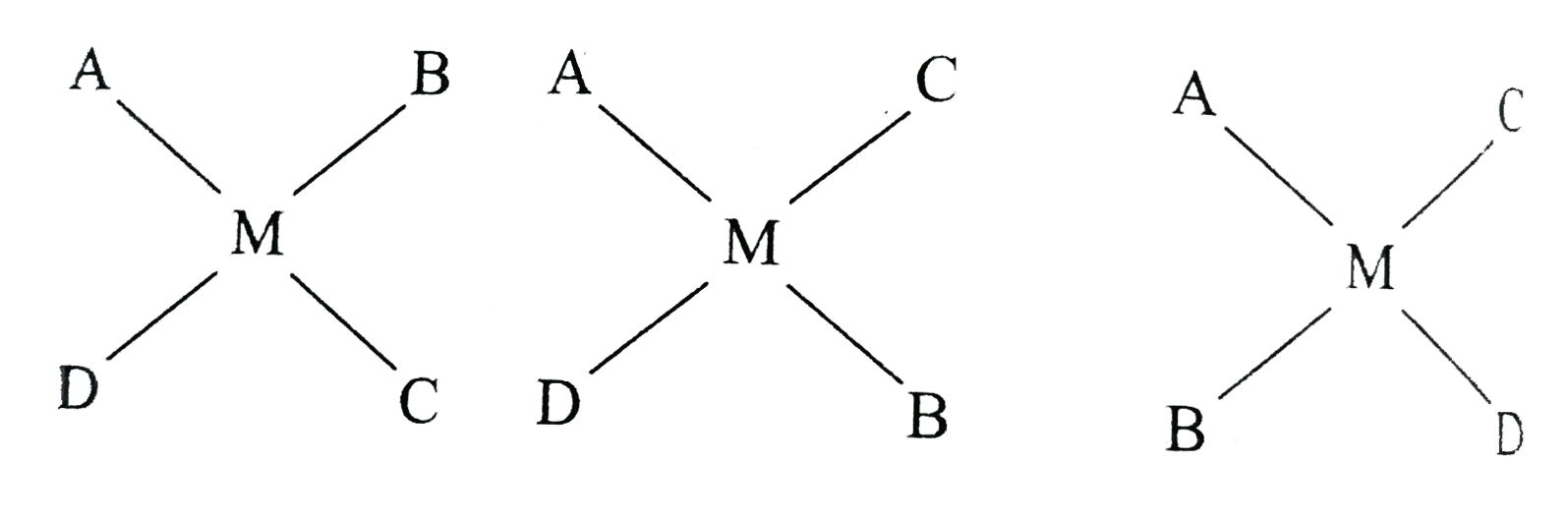
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
A2Z|Exercise Optical Isomerism|22 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Wemer Theory And Vbt|42 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Nomenclature|23 VideosCHEMICAL KINETICS
A2Z|Exercise Section D - Chapter End Test|30 VideosELECTROCHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-COORDINATION COMPOUNDS-Structural Isomerism
- The type of isomerism shown by [Co(en)2(NCS)2]Cl and [Co(en)2(NCS)Cl]N...
Text Solution
|
- Which of the following square planar complexes will form two geometric...
Text Solution
|
- The square planar complex (MABCD) can show
Text Solution
|
- Out of the following which will not show geometrical isomerism?
Text Solution
|
- [Co(NH3)4Cl2]^(+) exhibits
Text Solution
|
- The Type of isomerism present in pentaammine nitro chromium(III) perch...
Text Solution
|
- The most stable ion is .
Text Solution
|
- Which of the following complex ions has geometrical isomers?
Text Solution
|
- Which of the following pairs represents linkage isomers?
Text Solution
|
- The number of geometrical isomers of [Co(NH3)3(NO3)3] are:
Text Solution
|
- A square planar complex is represented as:
Text Solution
|
- The two compounds sulphato penta-ammine cobalt (III) bromide and sulph...
Text Solution
|
- Cis-trans isomerism is found in square planar complexes of molecular f...
Text Solution
|
- Which one of the following is an example of coordination isomerism?
Text Solution
|
- A square planar complex represented as:
Text Solution
|
- A compound has the empirical formula CoCl(3).5NH3. When an aqueous sol...
Text Solution
|
- The isomer
Text Solution
|
- Which of the following octahedral complex does not show geometrical is...
Text Solution
|
- [Co(NH3)5NO2]Cl2 and [Co(NH3)5(ONO)Cl2] are related to each other as
Text Solution
|
- [Co(NH3)5Br]SO4 and [CO(NH3)5SO4]Br are examples of which type of isom...
Text Solution
|